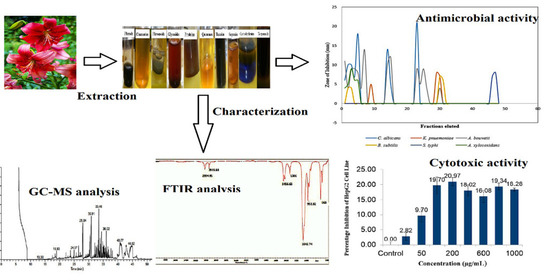
Lilium philadelphicum Flower as a Novel Source of Antimicrobial Agents: A Study of Bioactivity, Phytochemical Analysis, and Partial Identification of Antimicrobial Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of L. philadelphicum Flowers and Microbial Cultures
2.2. Purification and Characterization of Bioactive Compounds from the Red Lily Flower
2.3. Evaluating Antimicrobial Activity of the Red Lily Flower Extract
Phytochemical Screening
2.4. Spectroscopic Analyses of Active Fraction
2.5. Estimating Minimum Inhibitory Concentration (MIC) of the Partially Purified Compound
2.6. Analyzing Cytotoxicity Using MTT Assay
3. Results and Discussion
3.1. Antimicrobial and Phytochemical Screening of Solvent Extracts
3.2. Purification and Chemical Characterization of Bioactive Compound
3.3. Estimating Minimum Inhibitory Concentration (MIC) and Cytotoxicity of the Active Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. (GMD) 2012, 5, 1471–1492. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, C.R.; Ryves, D.B.; Millett, J. The function of secondary metabolites in plant carnivory. Ann. Bot. 2020, 14, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.; Wei, Q. Trends and applications in plant volatile sampling and analysis. Plant. J. 2021, 106, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Chory, J.; Dangl, J.L.; Estelle, M.; Jacobsen, S.E.; Meyerowitz, E.M.; Nordborg, M.; Weigel, D. The Impact of Arabidopsis on Human Health: Diversifying Our Portfolio. Cell 2008, 133, 939–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Singh, V.; Maurya, S.K.; Kamal, M.A.; Poddar, K.N. Antimicrobial and Antifungal Properties of Leaves to Root Extracts and Saponin Fractions of Chlorophytum borivilianum. Curr. Bioact. Compd. 2020, 16, e010621186650. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.; Farnsworth, B. The potential of alkaloids in drug discovery. Phyther. Res. 2001, 15, 183–205. [Google Scholar]
- Zhao, G.H.; Li, Z.X.; Chen, Z.D.; Xuebao, W.Q.D. Chemical structure and antitumor activity of polysaccharide from Lilium brownie. J. Food Sci. Biotechnol. 2002, 21, 62–66. [Google Scholar]
- Wang, H.; Ng, T.B. Isolation of lilin, a novel arginine- and glutamate-rich protein with potent antifungal and mitogenic activities from lily bulbs. Life Sci. 2002, 70, 1075–1084. [Google Scholar] [CrossRef]
- Francis, J.A.; Rumbeiha, W.; Nair, M.G. Constituents in Easter lily flowers with medicinal activity. Life Sci. 2004, 76, 671–683. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ibegbulem, C.O.; Mbagwu, F.N. Bioactive Principles from Medicinal Plants. Res. J. Phytochem. 2015, 9, 88–115. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga, L.L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement Altern. Med. 2001, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Malik, T.; Ibrahim, I.; Nadeem, S.G. Phytochemical screening and antibacterial activity of neem extracts on uropathogens. Pure Appl. Biol. 2002, 9, 148–153. [Google Scholar] [CrossRef]
- Adedayo, O.; Anderson, W.A.; Moo-Young, M.; Snieckus, V.; Patil, P.A.; Kolawole, D.O. Phytochemistry and antibacterial activity of Senna alata Flower. Pharm. Biol. 2001, 39, 408–412. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, R.L.; Gupta, A. Antioxidant, DNA protective and antibacterial activities of Terminalia bellerica extracts. J. Med. Plants Res. 2019, 13, 431–442. [Google Scholar]
- Dontha, M.B.; Kamurthy, S.H. Phytochemical Characterization of Active Constituents from Extracts of Ixora Javanica DC Flowers. J. Chromatogr. Sep. Tech. 2015, 6, 1–5. [Google Scholar]
- Soliman, S.; Alnajdy, D.; El-Keblawy, A.; Mosa, K.; Khoder, G.; Noreddin, A. Plants’ natural products as alternative promising anti-Candida drugs. Pharmacogn. Rev. 2017, 11, 104–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Singh, V.; Tripathi, C.K.M.; Bihari, V. Production, optimization and purification of an antifungal compound from Streptomyces capoamus MTCC 8123. Med. Chem. Res. 2008, 17, 94–102. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Montalvão, I.G.H.M.; Singh, V.; Haque, S. Bioassays for bioactivity screening. In Analysis of Marine Samples in Search of Bioactive Compounds; Elsevier BV: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Mandal, S.; Patra, A.; Samanta, A.; Roy, S.; Mandal, A.; Das Mahapatra, T.; Pradhan, S.; Das, K.; Nandi, D.K. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac. J. Trop. Biomed. 2013, 3, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.S.; Venkateshwar, C.; Sameul, G.; Rao, G.S. Phytochemical screening of some compounds from plant leaf extracts of Holoptelea integrifolia (planch.) and Celestrus emarginata (Grah.) used by Gondu tribes at Adilabad District, Andhra Pradesh, India. Int. J. Eng. Sci. Invent. 2013, 2, 65–70. [Google Scholar]
- Yadav, M.; Chatterji, S.; Gupta, S.K.; Watal, G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014, 6, 539–542. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Approved Standard M7-A5 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 4th ed.; NCCLS: Wayne, PA, USA, 2000.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]





| Compounds | Extract | |||
|---|---|---|---|---|
| Aqueous | Chloroform | Methanol | Hexane | |
| Saponins | − | − | − | − |
| Phenols | + | + | + | − |
| Glycosides | + | + | + | + |
| Flavonoids | + | + | + | + |
| Carbohydrates | − | − | − | − |
| Proteins | − | − | − | − |
| Coumarins | + | + | + | − |
| Quinones | + | − | + | − |
| Tannins | + | − | + | − |
| Terpenoids | + | − | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Singh, V.; Alhazmi, A.; Mishra, B.N.; Haque, S.; Sayyed, R.Z.; Sunita, K. Lilium philadelphicum Flower as a Novel Source of Antimicrobial Agents: A Study of Bioactivity, Phytochemical Analysis, and Partial Identification of Antimicrobial Metabolites. Sustainability 2021, 13, 8471. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158471
Singh S, Singh V, Alhazmi A, Mishra BN, Haque S, Sayyed RZ, Sunita K. Lilium philadelphicum Flower as a Novel Source of Antimicrobial Agents: A Study of Bioactivity, Phytochemical Analysis, and Partial Identification of Antimicrobial Metabolites. Sustainability. 2021; 13(15):8471. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158471
Chicago/Turabian StyleSingh, Shefali, Vineeta Singh, Alaa Alhazmi, Bhartendu Nath Mishra, Shafiul Haque, R. Z. Sayyed, and Kumari Sunita. 2021. "Lilium philadelphicum Flower as a Novel Source of Antimicrobial Agents: A Study of Bioactivity, Phytochemical Analysis, and Partial Identification of Antimicrobial Metabolites" Sustainability 13, no. 15: 8471. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158471