Microaerobic Digestion of Low-Biodegradable Sewage Sludge: Effect of Air Dosing in Batch Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analytical Methods
2.2.1. Gas-Phase Analyses
2.2.2. Liquid-Phase Analyses
2.2.3. Solid-Phase Analyses
3. Results and Discussion
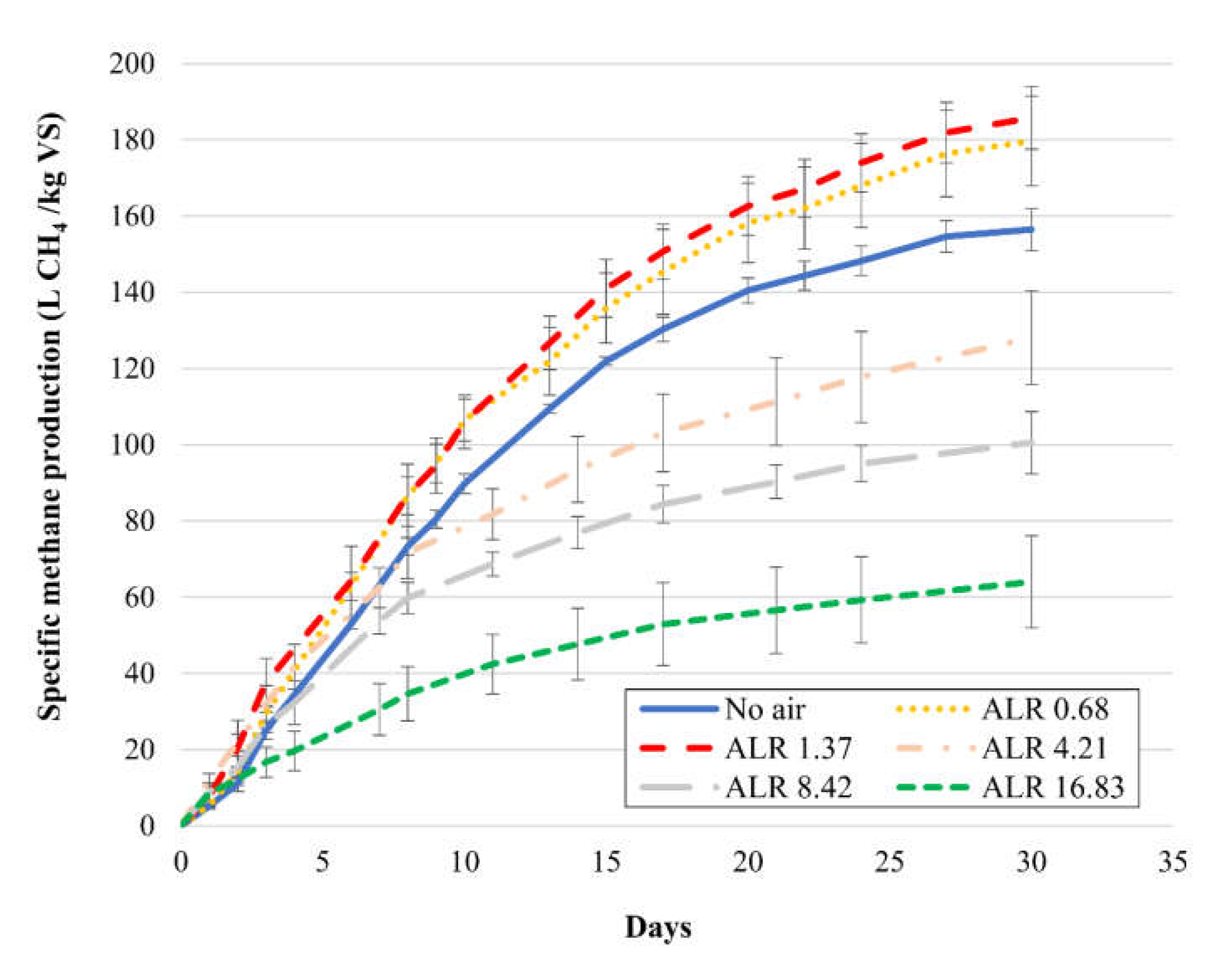
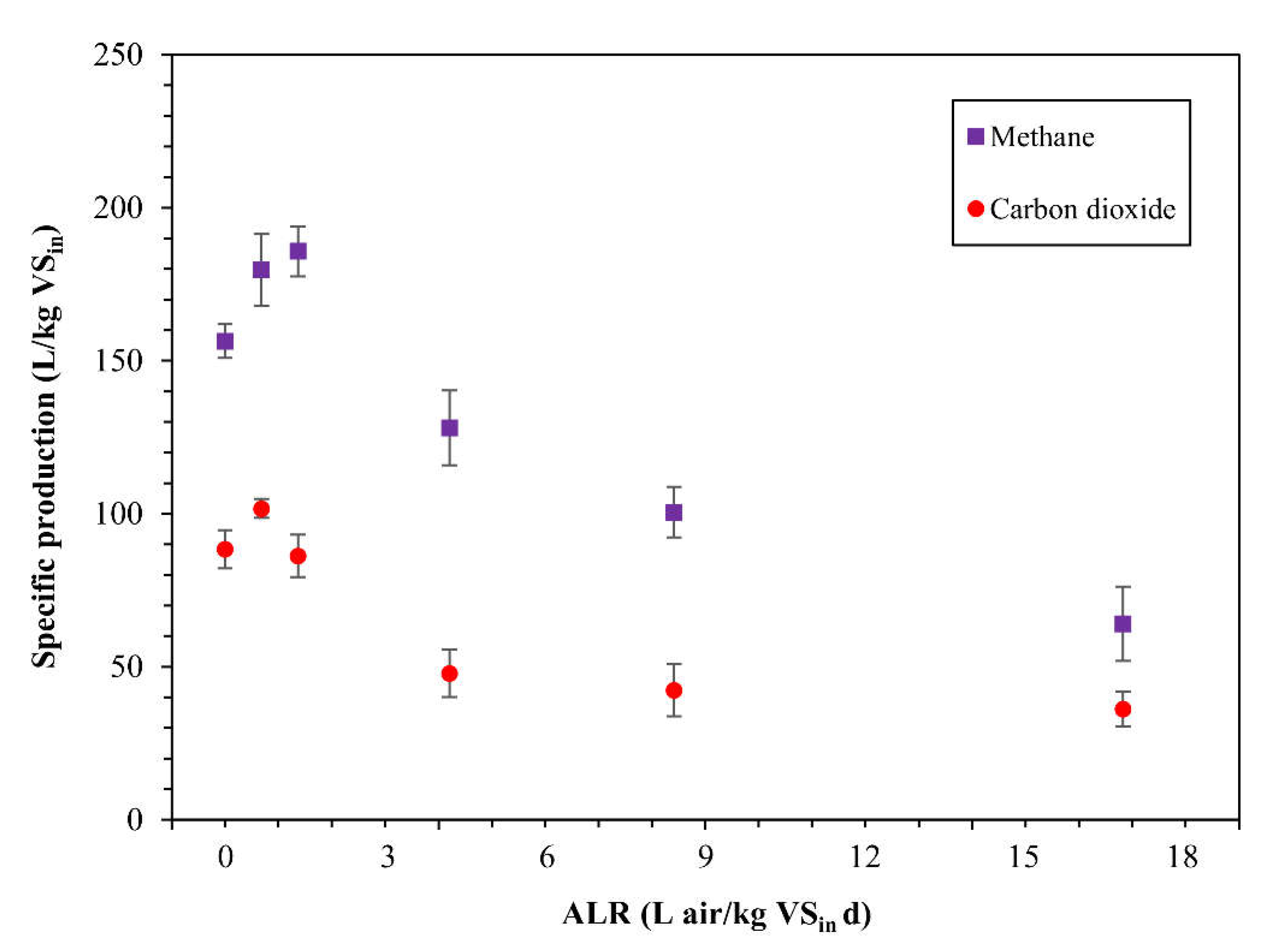
3.1. Effect of Different Air Doses on Methane Production
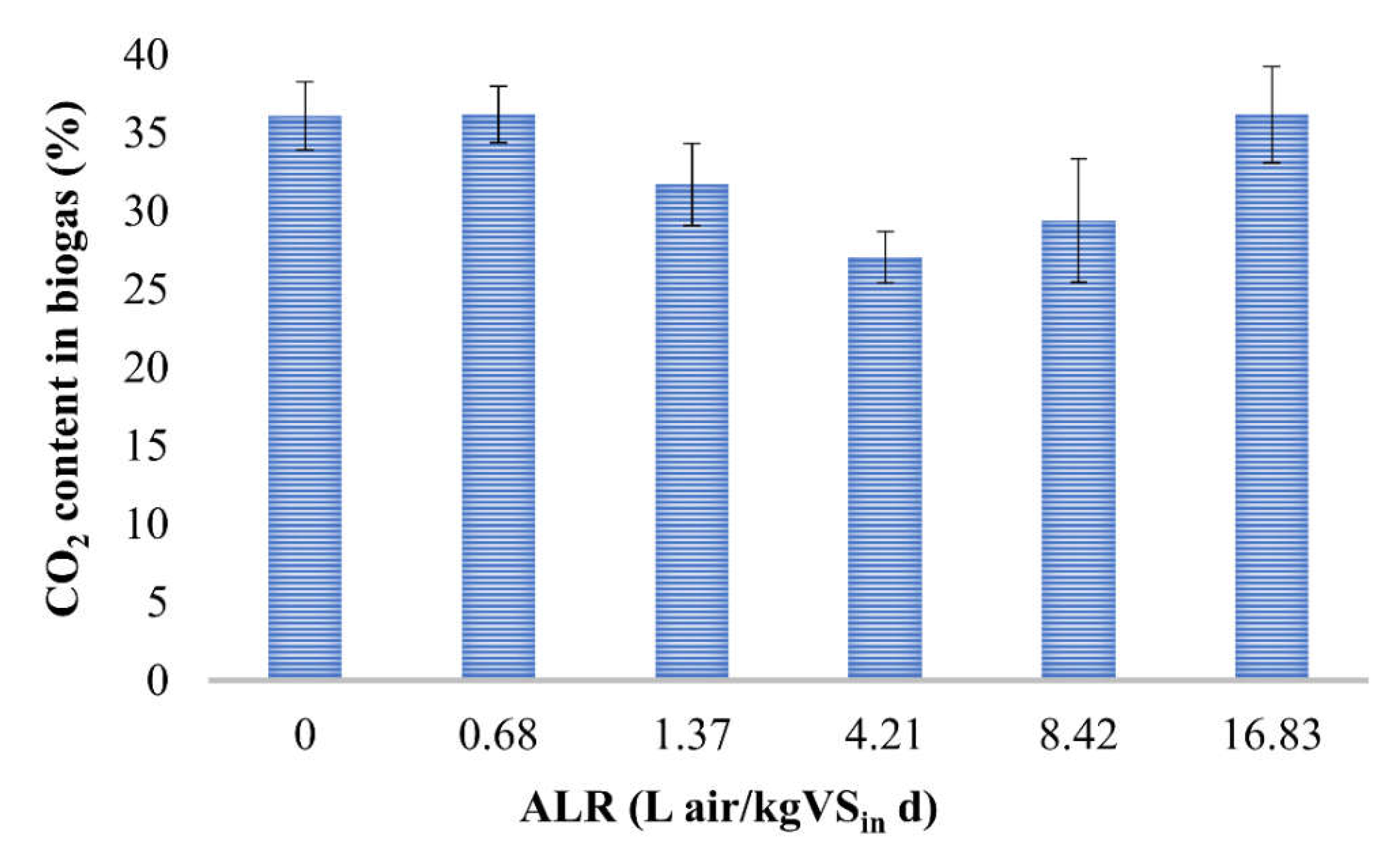
3.2. Effect of Microaeration on Biogas Composition
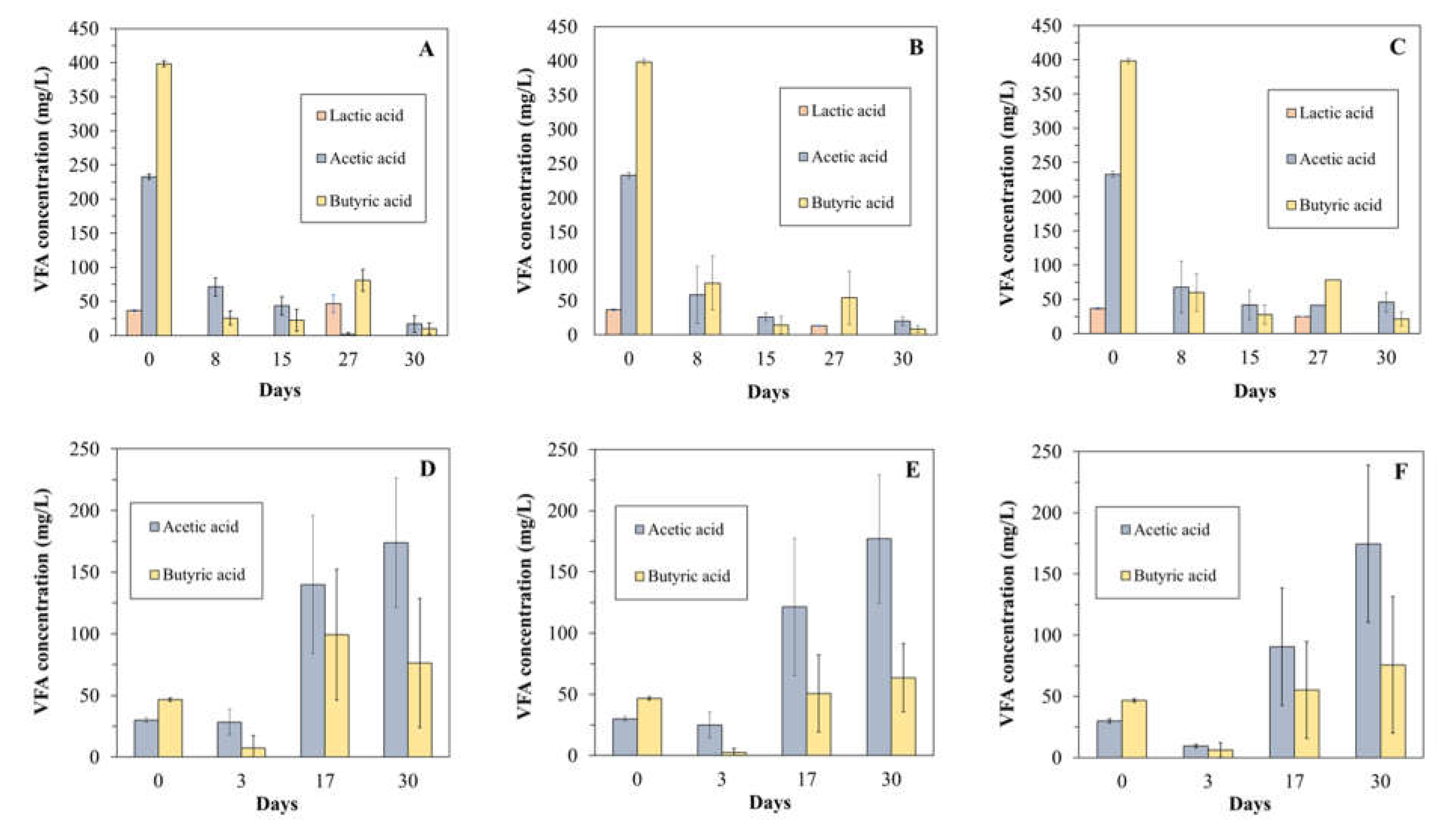
3.3. Impact of Different Air Doses on VFA Accumulation
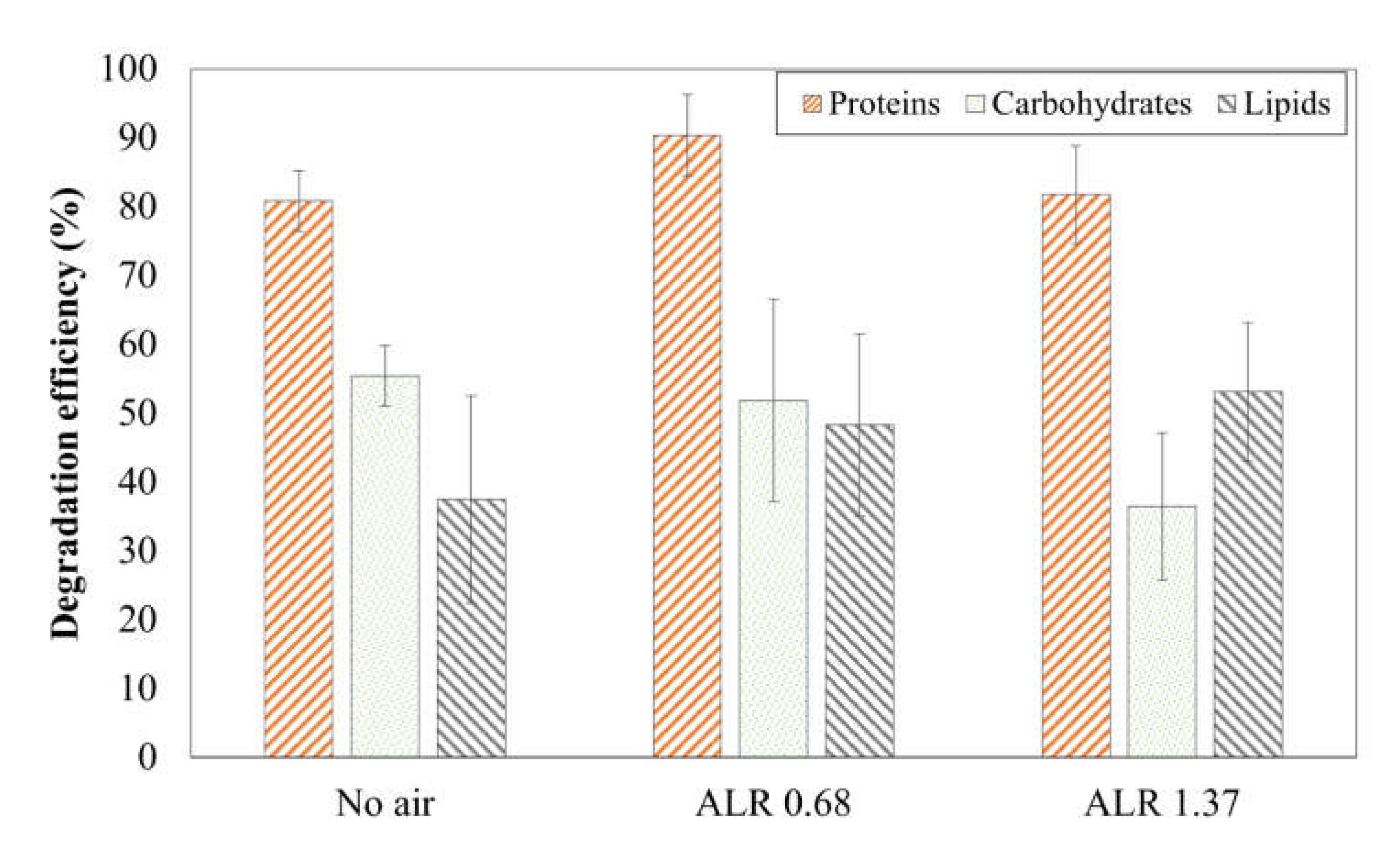
3.4. Influence of Microaeration on Degradation and Humification of SS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-solid anaerobic digestion of sewage sludge: Challenges and opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Nowak, O. Optimizing the use of sludge treatment facilities at municipal WWTPs. J. Environ. Sci. Health A 2006, 41, 1807–1817. [Google Scholar] [CrossRef]
- Kelley, E.; Twohig, E. Wastewater Treatment Sludge and Septage Management in Vermont; Vermont Department of Environmental Conservation: Essex Junction, VT, USA, 2018. [Google Scholar]
- Maqbool, T.; Cho, J.; Hur, J. Spectroscopic descriptors for dynamic changes of soluble microbial products from activated sludge at different biomass growth phases under prolonged starvation. Water Res. 2017, 123, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Pontoni, L.; Roviello, V.; Race, M.; Savignano, L.; van Hullebusch, E.D.; Esposito, G.; Pirozzi, F.; Fabbricino, M. Supramolecular aggregation of colloidal natural organic matter masks priority pollutants released in water from peat soil. Environ. Res. 2021, 195, 110761. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Modeling effects of DO and SRT on activated sludge decay and production. Water Res. 2015, 80, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Poquet, V.; Papirio, S.; Steyer, J.P.; Trably, E.; Escudié, R.; Esposito, G. High-solids anaerobic digestion model for homogenized reactors. Water Res. 2018, 142, 501–511. [Google Scholar] [CrossRef]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Nah, I.W.; Kang, Y.W.; Hwang, K.Y.; Song, W.K. Mechanical pretreatment of waste activated sludge for anaerobic digestion process. Water Res. 2000, 34, 2362–2368. [Google Scholar] [CrossRef]
- Lee, I.; Han, J.I. The effects of waste-activated sludge pretreatment using hydrodynamic cavitation for methane production. Ultrason. Sonochem. 2013, 20, 1450–1455. [Google Scholar] [CrossRef]
- Show, K.Y.; Mao, T.; Lee, D.J. Optimisation of sludge disruption by sonication. Water Res. 2007, 41, 4741–4747. [Google Scholar] [CrossRef]
- Chu, L.; Yan, S.; Xing, X.-H.; Sun, X.; Jurcik, B. Progress and perspectives of sludge ozonation as a powerful pretreatment method for minimization of excess sludge production. Water Res. 2009, 43, 1811–1822. [Google Scholar] [CrossRef]
- Ding, H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Di Capua, F.; Adani, F.; Pirozzi, F.; Esposito, G.; Giordano, A. Air side-stream ammonia stripping in a thin film evaporator coupled to high-solid anaerobic digestion of sewage sludge: Process performance and interactions. J. Environ. Manag. 2021, 295, 113075. [Google Scholar] [CrossRef]
- Giordano, A.; Di Capua, F.; Esposito, G.; Pirozzi, F. Long-term biogas desulfurization under different microaerobic conditions in full-scale thermophilic digesters co-digesting high-solid sewage sludge. Int. Biodeterior. Biodegrad. 2019, 142, 131–136. [Google Scholar] [CrossRef]
- Ramos, I.; Pérez, R.; Fdz-Polanco, M. Microaerobic desulphurisation unit: A new biological system for the removal of H2S from biogas. Bioresour. Technol. 2013, 142, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Jeníček, P.; Keclik, F.; Maca, J.; Bindzar, J. Use of microaerobic conditions for the improvement of anaerobic digestion of solid wastes. Water Sci. Technol. 2008, 58, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jeníček, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Jeníček, P.; Celis, C.A.; Krayzelova, L.; Anferova, N.; Pokorna, D. Improving products of anaerobic sludge digestion by microaeration. Water Sci. Technol. 2014, 69, 803–809. [Google Scholar] [CrossRef]
- Pontoni, L.; Papirio, S.; D’Alessandro, G.; Caniani, D.; Gori, R.; Mannina, G.; Capodici, M.; Nicosia, S.; Fabbricino, M.; Pirozzi, F.; et al. Dewaterability of CAS and MBR Sludge: Effect of Biological Stability and EPS Composition. J. Environ. Eng. 2018, 144, 04017088. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Palacios, T.; Ruiz, J.; Martín, D.; Muriel, E.; Antequera, T. Comparison of different methods for total lipid quantification in meat and meat products. Food Chem. 2008, 110, 1025–1029. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- De Nobili, M.; Cercignani, G.; Leita, L. Evaluation of type and contents of humic substances in sludges and composts. In Long-term Effects of Sewage Sludge and Farm Slurries Applications; Williams, J.H., Guidi, G., L’Hermite, P., Eds.; CRC Press: Boca Raton, FL, USA, 1985; pp. 181–186. [Google Scholar]
- Angelova, V.R.; Akova, V.I.; Ivanov Penka, A.; Licheva, K.I. Comparative study of titrimetric methods for determination of organic carbon in soils, compost and sludge. Ecol. Saf. 2014, 8, 430–440. [Google Scholar]
- Tang, Y.; Shigematsu, T.; Morimura, S.; Kida, K. The effects of micro-aeration on the phylogenetic diversity of microorganisms in a thermophilic anaerobic municipal solid-waste digester. Water Res. 2004, 38, 2537–2550. [Google Scholar] [CrossRef]
- Johansen, J.-E.; Bakke, R. Enhancing hydrolysis with microaeration. Water Sci. Technol. 2006, 53, 43–50. [Google Scholar] [CrossRef]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.M.; Wi, J.; Park, J.K.; Higuchi, S.; Lee, N.H. Effects of pre-aeration on the anaerobic digestion of sewage sludge. Environ. Eng. Res. 2014, 19, 59–66. [Google Scholar] [CrossRef]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Duarte, M.S.; Silva, S.A.; Salvador, A.F.; Cavaleiro, A.J.; Stams, A.J.M.; Alves, M.M.; Pereira, M.A. Insight into the Role of Facultative Bacteria Stimulated by Microaeration in Continuous Bioreactors Converting LCFA to Methane. Environ. Sci. Technol. 2018, 52, 6497–6507. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.W.; Chiam, J.A.; Wang, J.Y. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Liu, R.; Hao, X.; van Loosdrecht, M.C.M.; Zhou, P.; Li, J. Dynamics of humic substance composition during anaerobic digestion of excess activated sludge. Int. Biodeterior. Biodegrad. 2019, 145, 104771. [Google Scholar] [CrossRef]
- Yap, S.D.; Astals, S.; Lu, Y.; Peces, M.; Jensen, P.D.; Batstone, D.J.; Tait, S. Humic acid inhibition of hydrolysis and methanogenesis with different anaerobic inocula. Waste Manag. 2018, 80, 130–136. [Google Scholar] [CrossRef] [PubMed]

| pH | Carbonate Alkalinity (mg CaCO3/L) | TS (g/L) | VS (g/L) | Proteins (mg/L) | Carbohydrates (mg/L) | Lipids (mg/L) | HS (mg/gVS) |
|---|---|---|---|---|---|---|---|
| 7.63 | 1065 | 29.2 (±6.8) | 16.0 (±1.4) | 3943 (±647) | 248 (±25) | 1909 (±291) | 118 (±21) |
| Air Dose (mL) | ALR (L air/kg VSin d) | SMP (L CH4/kg VSin) | SCP (L CO2/kg VSin) | CH4 (%) | CH4/CO2 |
|---|---|---|---|---|---|
| 0 | 0 | 156 (±6) | 88 (±6) | 64 (±2) | 1.8 |
| 5 | 0.68 | 180 (±12) | 102 (±3) | 64 (±2) | 1.8 |
| 10 | 1.37 | 186 (±8) | 86 (±7) | 68 (±3) | 2.2 |
| 50 | 4.21 | 128 (±12) | 48 (±8) | 73 (±1) | 2.7 |
| 100 | 8.42 | 101 (±8) | 42 (±9) | 71 (±4) | 2.4 |
| 200 | 16.83 | 64 (±12) | 36 (±6) | 64 (±3) | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morello, R.; Di Capua, F.; Pontoni, L.; Papirio, S.; Spasiano, D.; Fratino, U.; Pirozzi, F.; Esposito, G. Microaerobic Digestion of Low-Biodegradable Sewage Sludge: Effect of Air Dosing in Batch Reactors. Sustainability 2021, 13, 9869. https://0-doi-org.brum.beds.ac.uk/10.3390/su13179869
Morello R, Di Capua F, Pontoni L, Papirio S, Spasiano D, Fratino U, Pirozzi F, Esposito G. Microaerobic Digestion of Low-Biodegradable Sewage Sludge: Effect of Air Dosing in Batch Reactors. Sustainability. 2021; 13(17):9869. https://0-doi-org.brum.beds.ac.uk/10.3390/su13179869
Chicago/Turabian StyleMorello, Raffaele, Francesco Di Capua, Ludovico Pontoni, Stefano Papirio, Danilo Spasiano, Umberto Fratino, Francesco Pirozzi, and Giovanni Esposito. 2021. "Microaerobic Digestion of Low-Biodegradable Sewage Sludge: Effect of Air Dosing in Batch Reactors" Sustainability 13, no. 17: 9869. https://0-doi-org.brum.beds.ac.uk/10.3390/su13179869






