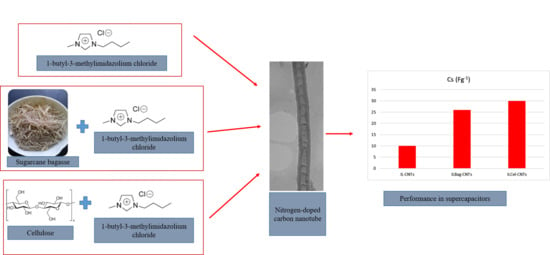
Effects of Ionic Liquid and Biomass Sources on Carbon Nanotube Physical and Electrochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussions
3.1. Morphological Analysis
3.2. Chemical Composition Analysis
3.3. Thermal Analysis
3.4. Surface Area and Porosity Analysis
3.5. Cyclic Voltammetry
3.6. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.A.; Bremner, S.P. Energy conversion approaches and materials for high-efficiency photovoltaics. Nat. Mater. 2016, 16, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Guo, L. Nature-inspired electrochemical energy-storage materials and devices. Adv. Energy Mater. 2017, 7, 1601709. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Bairi, P.; Shrestha, R.G.; Hill, J.P.; Nishimura, T.; Ariga, K.; Shrestha, L.K. Mesoporous graphitic carbon microtubes derived from fullerene C 70 tubes as a high performance electrode material for advanced supercapacitors. J. Mater. Chem. A 2016, 4, 13899–13906. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Huang, J.-Q.; Qian, W.-Z.; Zhang, Y.-Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Fic, K.; Frackowiak, E.; Béguin, F. Unusual energy enhancement in carbon-based electrochemical capacitors. J. Mater. Chem. 2012, 22, 24213–24223. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Mugadza, K.; Ndungu, P.G.; Stark, A.; Nyamori, V.O. Ionic liquids and cellulose: Innovative feedstock for synthesis of carbon nanostructured material. Mater. Chem. Phys. 2019, 234, 201–209. [Google Scholar] [CrossRef]
- Labulo, A.H.; Ngidi, N.P.; Omondi, B.; Nyamori, V.O. Physicochemical properties of nitrogen-doped carbon nanotubes from metallocenes and ferrocenyl imidazolium compounds. J. Organomet. Chem. 2018, 868, 66–75. [Google Scholar] [CrossRef]
- Steinmetz, M.; Lima, D.; Machado RR, L.; Sundararaj, U.; Arjmand, M.; da Silva, A.B.; Santos, J.P.; Pessôa, C.A.; Wohnrath, K. Nitrogen-doped carbon nanotubes towards electrochemical sensing: Effect of synthesis temperature. Diam. Relat. Mater. 2020, 110, 108093. [Google Scholar] [CrossRef]
- Mori, S.; Suzuki, M. Effect of oxygen and hydrogen addition on the low-temperature synthesis of carbon nanofibers using a low-temperature CO/Ar DC plasma. Diam. Relat. Mater. 2008, 17, 999–1002. [Google Scholar] [CrossRef]
- Hassan, F.M.; Chabot, V.; Li, J.; Kim, B.K.; Ricardez-Sandoval, L.; Yu, A. Pyrrolic-structure enriched nitrogen-doped graphene for highly efficient next-generation supercapacitors. J. Mater. Chem. A 2013, 1, 2904–2912. [Google Scholar] [CrossRef]
- Biemolt, J.; Denekamp, I.M.; Slot, T.K.; Rothenberg, G.; Eisenberg, D. Boosting the supercapacitance of nitrogen-doped carbon by tuning surface functionalities. ChemSusChem 2017, 10, 4018–4024. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W.; Wang, C. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Li, H.F.; Wang, C.; Liu, L.F.; Xie, N.; Pan, M.; Wu, P.; Wang, X.D.; Zeng, Z.; Deng, S.; Dai, G.P. Facile one-step synthesis of N-doped carbon nanotubes/N-doped carbon nanofibers hierarchical composites by chemical vapor deposition. J. Nanoparticle Res. 2020, 22, 10. [Google Scholar] [CrossRef]
- Sing, K.; Williams, R. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorp. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Mohammadi, R.; Asadian, E. One-step fabrication of electrochemically reduced graphene oxide/nickel oxide composite for binder-free supercapacitors. Int. J. Hydrog. Energy 2016, 41, 17496–17505. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Lota, G.; Machnikowski, J.; Vix-Guterl, C.; Béguin, F. Optimisation of supercapacitors using carbons with controlled nanotexture and nitrogen content. Electrochim. Acta 2006, 51, 2209–2214. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kumar, K.; Fisher, F.T.; Yang, E.-H. Out-of-plane growth of CNTs on graphene for supercapacitor applications. J. Nanotechnol. 2011, 23, 015301. [Google Scholar] [CrossRef]
- Mombeshora, E.T.; Ndungu, P.G.; Jarvis, A.L.L.; Nyamori, V.O. Oxygen-modified multiwalled carbon nanotubes: Physicochemical properties and capacitor functionality. Int. J. Energy Res. 2017, 41, 1182–1201. [Google Scholar] [CrossRef]
- Qian, X.; Li, N.; Imerhasan, M.; Wang, W. Conversion of low molecular weight hydrogel to nitrogen-doped carbon materials and its application as supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 255–261. [Google Scholar] [CrossRef]
- Mombeshora, E.T.; Ndungu, P.G.; Jarvis, A.L.L.; Nyamori, V.O. The physical and electrochemical properties of nitrogen-doped carbon nanotube- and reduced graphene oxide-titania nanocomposites. Mater. Chem. Phys. 2018, 213, 102–112. [Google Scholar] [CrossRef]
- Han, X.; Jiang, H.; Zhou, Y.; Hong, W.; Zhou, Y.; Gao, P.; Ding, R.; Liu, E. A high performance nitrogen-doped porous activated carbon for supercapacitor derived from pueraria. J. Alloys Compd. 2018, 744, 544–551. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z. Recent breakthroughs in supercapacitors boosted by nitrogen-rich porous carbon materials. Adv. Sci. 2017, 4, 1600408. [Google Scholar] [CrossRef] [Green Version]
- Ewels, C.; Glerup, M. Nitrogen doping in carbon nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1345–1363. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-R.; Jun, H.; Jiang-Feng, L.; Ping, C. N-doped carbon nanotubes derived from waste biomass and its electrochemical performance. Mater. Let. 2020, 261, 127146. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Chen, Y.; Chen, Y.; Guo, H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Liou, T.-H.; Wu, S.-J. Characteristics of microporous/mesoporous carbons prepared from rice husk under base- and acid-treated conditions. J. Hazard. Mater 2009, 171, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, M.; Liu, Y.; Lu, T.; Pan, L. Metal-organic framework-engaged formation of a hierarchical hybrid with carbon nanotube inserted porous carbon polyhedra for highly efficient capacitive deionization. J. Mater. Chem. A 2016, 4, 5467–5473. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugadza, K.; Stark, A.; Ndungu, P.G.; Nyamori, V.O. Effects of Ionic Liquid and Biomass Sources on Carbon Nanotube Physical and Electrochemical Properties. Sustainability 2021, 13, 2977. https://0-doi-org.brum.beds.ac.uk/10.3390/su13052977
Mugadza K, Stark A, Ndungu PG, Nyamori VO. Effects of Ionic Liquid and Biomass Sources on Carbon Nanotube Physical and Electrochemical Properties. Sustainability. 2021; 13(5):2977. https://0-doi-org.brum.beds.ac.uk/10.3390/su13052977
Chicago/Turabian StyleMugadza, Kudzai, Annegret Stark, Patrick G. Ndungu, and Vincent O. Nyamori. 2021. "Effects of Ionic Liquid and Biomass Sources on Carbon Nanotube Physical and Electrochemical Properties" Sustainability 13, no. 5: 2977. https://0-doi-org.brum.beds.ac.uk/10.3390/su13052977