Remote Sensing of Leaf and Canopy Nitrogen Status in Winter Wheat (Triticum aestivum L.) Based on N-PROSAIL Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Acquisition
2.2.1. Canopy Spectral Data
2.2.2. Plant Measurements
2.3. Model and Methods
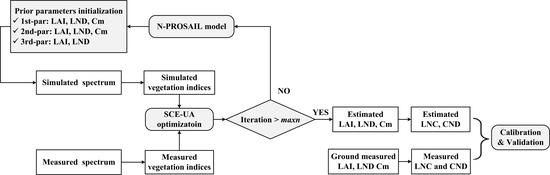
2.3.1. Inversion Procedure of LNC and CND Estimation
- (i)
- 1st-par: LAI, LND and Cm were optimized and the other six parameters in the N-PROSAIL model (Table 3) were set as fixed values in the whole growth period;
- (ii)
- 2nd-par: LAI, LND and Cm were optimized and the other six parameters in the N-PROSAIL model (Table 3) were set as fixed values at each growth stage;
- (iii)
2.3.2. The N-PROSAIL Model
2.3.3. Selection of Spectral Index
2.3.4. SCE-UA Algorithm for LNC and CND Estimation
2.3.5. Statistical Analysis
3. Results
3.1. Correlations among LAI, Cm, LND, LNC, and CND
3.2. Correlations between Agronomic Variables and Vegetation Indices
3.3. LAI, LND, and Cm Estimation Using the N-PROSAIL Model Inversion
3.4. LNC and CND Estimation Based on LAI, LND, and Cm
3.5. Comparison of the N-PROSAIL Model Method with the Vegetation Index Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, X.; Huang, Y.; Shang, G.; Zhou, C.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of Six Algorithms to Monitor Wheat Leaf Nitrogen Concentration. Remote Sens. 2015, 7, 14939–14966. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Karimi, Y.; Prasher, S.O.; Patel, R.M.; Kim, S.H. Application of support vector machine technology for weed and nitrogen stress detection in corn. Comput. Electron. Agric. 2006, 51, 99–109. [Google Scholar] [CrossRef]
- Miphokasap, P.; Honda, K.; Vaiphasa, C.; Souris, M.; Nagai, M. Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy. Remote Sens. 2012, 4, 1651–1670. [Google Scholar] [Green Version]
- Kokaly, R.F. Investigating a Physical Basis for Spectroscopic Estimates of Leaf Nitrogen Concentration. Remote Sens. Environ. 2001, 75, 153–161. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, Y.; Sui, Z.; Zhang, Q.Y.; Zhao, C.J. Mechanism Analysis of Leaf Biochemical Concentration by High Spectral Remote Sensing. J. Remote Sens. 2000, 4, 125–130. [Google Scholar]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident Detection of crop Water Stress, Nitrogen Status and Canopy Density Using Ground-Based Multispectral Data. Available online: https://naldc.nal.usda.gov/download/4190/PDF (accessed on 11 September 2018).
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Rodriguez, D.; Christensen, L.K.; Belford, R.; Sadras, V.O.; Clarke, T.R. Spectral and thermal sensing for nitrogen and water status in rainfed and irrigated wheat environments. Precis. Agric. 2006, 7, 233–248. [Google Scholar]
- Feng, W.; Yao, X.; Zhu, Y.; Tian, Y.C.; Cao, W.X. Monitoring leaf nitrogen status with hyperspectral reflectance in wheat. Eur. J. Agron. 2008, 28, 394–404. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.C.; Yao, X.; Yang, J.; Cao, W.X.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground-and space-based hyperspectral reflectance. Fuel Energy Abstr. 2011, 120, 299–310. [Google Scholar] [CrossRef]
- Yao, X.; Jia, W.; Si, H.; Guo, Z.; Tian, Y.; Liu, X.; Cao, W.; Zhu, Y. Exploring novel bands and key index for evaluating leaf equivalent water thickness in wheat using hyperspectra influenced by nitrogen. PLoS ONE 2014, 9, e96352. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, H.Y.; Zhang, Y.S.; Qi, S.L.; Heng, Y.R.; Guo, B.B.; Ma, D.Y.; Guo, T.C. Remote detection of canopy leaf nitrogen concentration in winter wheat by using water resistance vegetation indices from in-situ hyperspectral data. Field Crop. Res. 2016, 198, 238–246. [Google Scholar] [CrossRef]
- Elshikha, D.M.; Barnes, E.M.; Clarke, T.R.; Hunsaker, D.J.; Haberland, J.A.; Pinter, P.J.; Waller, P.M.; Thompson, T.L. Remote Sensing of Cotton Nitrogen Status Using the Canopy Chlorophyll Content Index (CCCI). Trans. Asabe 2008, 51, 73–82. [Google Scholar]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Ecarnot, M.; Compan, F.; Roumet, P. Assessing leaf nitrogen content and leaf mass per unit area of wheat in the field throughout plant cycle with a portable spectrometer. Field Crop. Res. 2013, 140, 44–50. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Wang, S.; Ma, Y.; Wei, Q.; Li, X.; Cong, R.; Ren, T. Methods for estimating leaf nitrogen concentration of winter oilseed rape (Brassica napus L.) using in situ, leaf spectroscopy. Ind. Crop. Prod. 2016, 91, 194–204. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M.; Wachowiak, M.P.; Flores-Verdugo, F. Relationship between Hyperspectral Measurements and Mangrove Leaf Nitrogen Concentrations. Remote Sens. 2013, 5, 891–908. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.G.; Zhao, C.J.; Wang, J.H.; Li, C.J.; Yang, X.D. Associating new spectral features from visible and near infrared regions with optimal combination principle to monitor leaf nitrogen concentration in barley. J. Infrared Millim. Waves 2013, 32, 351. [Google Scholar] [CrossRef]
- Xu, X.G.; Zhao, C.J.; Wang, J.H.; Zhang, J.C.; Song, X.Y. Using optimal combination method and in situ hyperspectral measurements to estimate leaf nitrogen concentration in barley. Precis. Agric. 2014, 15, 227–240. [Google Scholar] [CrossRef]
- Li, Z.; Nie, C.; Wei, C.; Xu, X.; Song, X.; Wang, J. Comparison of Four Chemometric Techniques for Estimating Leaf Nitrogen Concentrations in Winter Wheat (Triticum aestivum) Based on Hyperspectral Features. J. Appl. Spectrosc. 2016, 83, 240–247. [Google Scholar] [CrossRef]
- Atzberger, C. Object-based retrieval of biophysical canopy variables using artificial neural nets and radiative transfer models. Remote Sens. Environ. 2004, 93, 53–67. [Google Scholar] [CrossRef]
- Li, Z.; Jin, X.; Wang, J.; Yang, G.; Nie, C.; Xu, X.; Feng, H. Estimating winter wheat (Triticum aestivum) LAI and leaf chlorophyll content from canopy reflectance data by integrating agronomic prior knowledge with the PROSAIL model. Int. J. Remote Sens. 2015, 36, 2634–2653. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, C.; Pu, R.; Feng, H.; Li, Z.; Li, H.; Sun, C. Leaf nitrogen spectral reflectance model of winter wheat (Triticum aestivum) based on PROSPECT: simulation and inversion. J. Appl. Remote Sens. 2015, 9. [Google Scholar] [CrossRef]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: the SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Combal, B.; Baret, F.; Weiss, M.; Trubuil, A.; Mace, D.; Pragnere, A.; Myneni, R.; Knyazikhin, Y.; Wang, L. Retrieval of canopy biophysical variables from bidirectional reflectance: Using prior information to solve the ill-posed inverse problem. Remote Sens. Environ. 2002, 84, 1–15. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Liu, L.; Song, B.; Zhang, S.; Liu, X. A Novel Principal Component Analysis Method for the Reconstruction of Leaf Reflectance Spectra and Retrieval of Leaf Biochemical Contents. Remote Sens. 2017, 9. [Google Scholar] [CrossRef]
- Schepers, J.S.; Francis, D.D.; Thompson, M.T. Simultaneous determination of total C, total N, and 15N on soil and plant material 1. Commun. Soil Sci. Plant Anal. 1989, 20, 949–959. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Huang, W. Quantitative Remote Sensing of Agriculture: Theory and Application; Science Press: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Boogaard, H.L.; Diepen, C.A.; Rotter, R.P.; Cabrera, J.A.; Laar, H.H. User’s guide for the WOFOST 7.1 Crop Growth Simulation Model and WOFOST Control Center. Available online: http://library.wur.nl/WebQuery/wurpubs/309027 (accessed on 11 September 2018).
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Vina, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Smith, A.M.S. Using in-situ measurements to evaluate the new RapidEye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote Mapping of Standing Crop Biomass for Estimation of the Productivity of the Shortgrass Prairie. Available online: http://adsabs.harvard.edu/abs/1972rse.conf.1355P (accessed on 11 September 2018).
- Le Maire, G.; François, C.; Soudani, K.; Berveiller, D.; Pontailler, J.Y.; Bréda, N.; Genet, H.; Davi, H.; Dufrêne, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.W.; Keulemans, W.; Coppin, P.A. near-infrared narrow-waveband ratio to determine Leaf Area Index in orchards. Remote Sens. Environ. 2008, 112, 3762–3772. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; De Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. Angular Dependence of Maize and Sugar Beet VIs from Directional CHRIS/Proba Data. Available online: https://www.researchgate.net/publication/228413259_Angular_dependence_of_maize_and_sugar_beet_VIs_from_directional_CHRISProba_data (accessed on 11 September 2018).
- Steddom, K.; Heidel, G.; Jones, D.; Rush, C.M. Remote detection of rhizomania in sugar beets. Phytopathology 2003, 93, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.Y.; Gupta, V.K.; Sorooshian, S. Shuffled complex evolution approach for effective and efficient global minimization. J. Optim. Theory Appl. 1993, 76, 501–521. [Google Scholar] [CrossRef]
- Duan, Q.; Sorooshian, S.; Gupta, V.K. Optimal use of the SCE-UA global optimization method for calibrating watershed models. J. Hydrol. 1994, 158, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhao, Y.; Feng, X. A methodology for estimating Leaf Area Index by assimilating remote sensing data into crop model based on temporal and spatial knowledge. Chin. Geogr. Sci. 2013, 23, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Wang, J.; Bo, Y.; Chen, G.; Xue, H. Data Assimilation of MODIS and TM Observations into CERES-Maize Model to Estimate Regional Maize Yield. Available online: https://0-doi-org.brum.beds.ac.uk/10.1117/12.860315 (accessed on 11 September 2018).
- Wang, H.; Zhu, Y.; Li, W.; Cao, W.; Tian, Y. Integrating remotely sensed leaf area index and leaf nitrogen accumulation with RiceGrow model based on particle swarm optimization algorithm for rice grain yield assessment. J. Appl. Remote Sens. 2014, 8. [Google Scholar] [CrossRef]
- Huang, J.; Ma, H.; Su, W.; Zhang, X.; Huang, Y.; Fan, J.; Wu, W. Jointly assimilating MODIS LAI and ET products into the SWAP model for winter wheat yield estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 4060–4071. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Feret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; Le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Koetz, B.; Baret, F.; Poilvé, H.; Hill, J. Use of coupled canopy structure dynamic and radiative transfer models to estimate biophysical canopy characteristics. Remote Sens. Environ. 2005, 95, 115–124. [Google Scholar] [CrossRef]






| No. | Season | Cultivar | Treatments | Dataset |
|---|---|---|---|---|
| Exp. 1 | 2012–2013 | Nongda211, Zhongmai175, Jing9843, Zhongyou206 | N rate (kg N ha−1): 0, 110, 220, 440 | Calibration |
| Exp. 2 | 2013–2014 | Jing9843, Zhongmai175 | N rate (kg N ha−1): 0, 90, 180, 270; Irrigation rate (mm): 0, 146, 292 | Calibration |
| Exp. 3 | 2014–2015 | Jing9843, Zhongmai175 | N rate (kg N ha−1): 0, 90, 180, 270; Irrigation rate (mm): 0, 192, 384 | Validation |
| Exp. 4 | 2015–2016 | Lunxuan167, Jingdong18 | N rate (kg N ha−1): 18, 90, 180, 270; | Calibration |
| Growth Stage | Zadoks | Date | No. | LAI | Cm | LND | LNC | CND | Spectral |
|---|---|---|---|---|---|---|---|---|---|
| Exp. 1: 2012–2013 | |||||||||
| Stem elongation | 31 | 23 Apr. | 32 | ✓ | ✓ | ✓ | ✓ | ✓ | -- |
| Booting | 47 | 6 May | 32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anthesis | 65 | 19 May | 32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Milk development | 75 | 1 Jun. | 32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Exp. 2: 2013–2014 | |||||||||
| Stem elongation | 33 | 11 Apr. | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Booting | 45 | 20 Apr. | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anthesis | 65 | 7 May | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Milk development | 75 | 20 May | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Exp. 3: 2014–2015 | |||||||||
| Stem elongation | 31 | 14 Apr. | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Booting | 47 | 26 Apr. | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anthesis | 65 | 12 May | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Milk development | 75 | 26 May | 48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Exp. 4: 2015–2016 | |||||||||
| Stem elongation | 31 | 15 Apr. | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Booting | 47 | 29 Apr. | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anthesis | 65 | 11 May | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Milk development | 75 | 26 May | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Parameters | 1st-par Setting | 2nd-par/3rd-par Setting | |||
|---|---|---|---|---|---|
| Z.S. 3.1 | Z.S. 47 | Z.S. 65 | Z.S. 75 | ||
| Leaf N density (LND, µg·cm−2) | 60–230 | 75–230 | 70–200 | 80–200 | 60–200 |
| Leaf area index (LAI, m2·m−2) | 0.4–8.5 | 1.5–6.5 | 1.0–8.5 | 0.5–7.0 | 0.3–4.5 |
| Dry matter content (Cm, g·m−2) | 40 | 23–60 #; 40 ## | 28–45 #; 36 ## | 30–54 #; 39 ## | 30–70 #; 46 ## |
| Equivalent water content (Cw, cm) | 0.010 | 0.0138 | 0.0127 | 0.0098 | 0.0085 |
| Leaf structure parameter (Ns) | 1.5 | 1.24 | 1.07 | 1.03 | 1.16 |
| Leaf inclination distribution (LID, deg) | 50 | 50.5 | 47.2 | 45.0 | 43.8 |
| Soil brightness parameter (Rsoil) | 0.44 | 0.49 | 0.73 | 0.62 | 0.35 |
| Hot spot parameter (SL) | 0.15 | 0.24 | 0.10 | 0.12 | 0.21 |
| Solar zenith angle (θs, deg) | 45 | 30 | 25 | 22 | 18 |
| Vegetation Index | Formulation | Reference |
|---|---|---|
| CIred edge | (R750/R720) − 1 | Gitelson et al. [38] |
| GNDVI | (R750 − R550)/(R750 + R550) | Baret et al. [39] |
| MCARI/MTVI2 | MCARI/MTVI2; MCARI: (R700 − R670 − 0.2(R700 − R550))(R700/R670); MTVI2: 0.5(1.2(R800−R500)-2.5(R670 − R550))/ sqrt (2(R800 + 1)2 − (6R800 − 5sqrt(R670)) − 0.5) | Eitel et al. [40] |
| mND705 | (R750 - R705)/(R750 + R705 − 2R445) | Sims et al. [41] |
| MSR | (R800/R670 − 1)/sqrt(R800/R670 + 1) | Chen [42] |
| ND705 | (R750 − R705)/(R750 + R705) | Sims et al. [41] |
| NDVI | (R890 − R670)/(R890 + R670) | Pearson et al. [43] |
| NDLMA | (R2260 − R1490)/(R2260 + R1490) | Le Maire et al. [44] |
| DCNI | (R720 − R700)/(R700 − R670)/(R720 − R670 + 0.03) | Chen et al. [13] |
| WDRVI | (0.1R890 − R670)/(0.1R890 + R670) | Gitelson et al. [45] |
| sLAIDI | s(R1050 − R1250)/(R1050 + R1250), s = 5 | Delalieux et al. [46] |
| GI | R551/R677 | Zarco-Tejada et al. [47] |
| SPVI | 0.4(3.7(R800 − R670) − 1.2abs(R550 − R670)) | Vincini et al. [48] |
| NDVIcanste | (R760 − R708)/(R760 + R708) | Steddom et al. [49] |
| NDRE | (R790 − R720)/(R790 + R720) | Fitzgerald et al. [12] |
| Variable | LAI | Cm | LND | LNC |
|---|---|---|---|---|
| Cm | −0.55 ** | |||
| LND | 0.22 ** | 0.45 ** | ||
| LNC | 0.66 ** | -0.19 ** | 0.73 ** | |
| CND | 0.90 ** | -0.30 ** | 0.52 ** | 0.84 ** |
| Variable | LAI | Cm | LND | LNC | CND |
|---|---|---|---|---|---|
| CIred edge | 0.76 ** | −0.22 ** | 0.49 ** | 0.70 ** | 0.81 ** |
| GNDVI | 0.77 ** | −0.21 ** | 0.49 ** | 0.70 ** | 0.79 ** |
| MCARI/MTVI2 | −0.69 ** | 0.14 ** | −0.56 ** | −0.71 ** | −0.75 ** |
| mND705 | 0.74 ** | −0.21 ** | 0.52 ** | 0.73 ** | 0.78 ** |
| MSR | 0.80 ** | −0.28 ** | 0.41 ** | 0.67 ** | 0.81 ** |
| ND705 | 0.76 ** | −0.23 ** | 0.50 ** | 0.71 ** | 0.79 ** |
| NDVI | 0.74 ** | −0.23 ** | 0.47 ** | 0.68 ** | 0.75 ** |
| NDLMA | 0.12* | −0.26 ** | −0.19 ** | 0.00 # | 0.06 # |
| DCNI | 0.40 ** | −0.01 # | 0.45 ** | 0.48 ** | 0.49 ** |
| WDRVI | 0.79 ** | −0.27 ** | 0.44 ** | 0.68 ** | 0.80 ** |
| sLAIDI | 0.68 ** | −0.20 ** | 0.38 ** | 0.56 ** | 0.69 ** |
| GI | 0.79 ** | −0.35 ** | 0.30 ** | 0.61 ** | 0.76 ** |
| SPVI | 0.79 ** | −0.28 ** | 0.44 ** | 0.71 ** | 0.82 ** |
| NDVIcanste | 0.76 ** | −0.22 ** | 0.51 ** | 0.72 ** | 0.79 ** |
| NDRE | 0.74 ** | −0.19 ** | 0.52 ** | 0.71 ** | 0.79 ** |
| Parameter Setting | Statistical Categories | No. | LAI (m2·m−2) | Cm (g·m−2) | LND (µg·cm−2) | LNC (%) | CND (g·m−2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |||
| 1st-par | Z.S.31 | 72 | 0.45 ** | 0.76 | 0.02 # | 26.43 | 0.29 ** | 42.24 | 0.00 # | 1.10 | 0.36 ** | 1.66 |
| Z.S.47 | 104 | 0.56 ** | 0.93 | 0.07 # | 25.50 | 0.48 ** | 70.07 | 0.49 ** | 1.99 | 0.69 ** | 3.19 | |
| Z.S.65 | 104 | 0.69 ** | 0.66 | 0.00 # | 21.84 | 0.42 ** | 64.42 | 0.47 ** | 2.32 | 0.74 ** | 2.61 | |
| Z.S.75 | 104 | 0.67 ** | 0.56 | 0.00 # | 21.86 | 0.25 ** | 48.33 | 0.11 ** | 0.64 | 0.68 ** | 1.78 | |
| Calibration | 384 | 0.67 ** | 0.74 | 0.00 # | 23.79 | 0.30 ** | 58.49 | 0.21 ** | 1.69 | 0.66 ** | 2.45 | |
| Validation | 192 | 0.76 ** | 0.93 | 0.00 # | 27.93 | 0.34 ** | 62.86 | 0.18 ** | 1.23 | 0.76 ** | 1.47 | |
| 2nd-par | Z.S.31 | 72 | 0.45 ** | 0.82 | 0.00 # | 18.51 | 0.34 ** | 18.53 | 0.03 # | 1.24 | 0.20 ** | 2.16 |
| Z.S.47 | 104 | 0.50 ** | 1.46 | 0.06 # | 8.21 | 0.61 ** | 18.60 | 0.38 ** | 0.52 | 0.68 ** | 2.57 | |
| Z.S.65 | 104 | 0.68 ** | 1.15 | 0.04 # | 11.85 | 0.51 ** | 17.57 | 0.21 ** | 0.65 | 0.73 ** | 2.15 | |
| Z.S.75 | 104 | 0.67 ** | 0.61 | 0.00 # | 22.68 | 0.25 ** | 26.55 | 0.02 # | 1.00 | 0.68 ** | 1.24 | |
| Calibration | 384 | 0.67 ** | 1.08 | 0.05 # | 16.12 | 0.45 ** | 20.80 | 0.14 ** | 0.87 | 0.67 ** | 2.08 | |
| Validation | 192 | 0.81 ** | 0.64 | 0.18 ** | 18.50 | 0.39 ** | 24.15 | 0.51 ** | 0.94 | 0.82 ** | 1.03 | |
| 3rd-par | Z.S.31 | 72 | 0.59 ** | 0.57 | 0.00 # | 4.81 | 0.57 ** | 15.16 | 0.34 ** | 0.41 | 0.44 ** | 1.31 |
| Z.S.47 | 104 | 0.67 ** | 0.88 | 0.00 # | 6.35 | 0.65 ** | 17.29 | 0.64 ** | 0.65 | 0.76 ** | 1.52 | |
| Z.S.65 | 104 | 0.70 ** | 0.81 | 0.00 # | 6.09 | 0.56 ** | 16.14 | 0.74 ** | 0.47 | 0.76 ** | 1.40 | |
| Z.S.75 | 104 | 0.66 ** | 0.55 | 0.00 # | 6.64 | 0.48 ** | 20.08 | 0.67 ** | 0.30 | 0.69 ** | 1.00 | |
| Calibration | 384 | 0.75 ** | 0.73 | 0.13 ** | 6.10 | 0.59 ** | 17.43 | 0.62 ** | 0.48 | 0.75 ** | 1.32 | |
| Validation | 192 | 0.80 ** | 0.69 | 0.47 ** | 8.19 | 0.46 ** | 21.18 | 0.75 ** | 0.38 | 0.82 ** | 0.95 | |
| Vegetation Index | LNC Model # | R2 | RMSE (%) | CND Model | R2 | RMSE (g·m−2) |
|---|---|---|---|---|---|---|
| CIred edge | y = 3.224x0.274 | 0.58 | 0.52 | y = 3.005x1.107 | 0.79 | 1.34 |
| GNDVI | y = 4.654x0.837 | 0.54 | 0.51 | y = 13.583x3.464 | 0.78 | 1.32 |
| MCARI/MTVI2 | y = 4.802e-5.938x | 0.56 | 0.48 | y = 14.41e23.28x | 0.73 | 1.38 |
| mND705 | y = 4.320x0.589 | 0.58 | 0.49 | y = 0.206e4.230 | 0.80 | 1.26 |
| MSR | y = 2.550x0.274 | 0.53 | 0.55 | y = 1.121x1.134 | 0.78 | 1.34 |
| ND705 | y = 1.870e0.977x | 0.57 | 0.52 | y = 0.325e4.061x | 0.80 | 1.26 |
| NDVI | y = 1.399e1.087x | 0.52 | 0.55 | y = 0.0925e4.518x | 0.77 | 1.34 |
| DCNI | y = 0.935x0.411 | 0.27 | 0.52 | y = 0.894e0.0604x | 0.30 | 1.73 |
| WDRVI | y = 1.345x + 3.335 | 0.48 | 0.55 | y = 3.181e1.738x | 0.75 | 1.33 |
| sLAIDI | y = 2.648e0.415x | 0.35 | 0.65 | y = 1.226e1.827x | 0.59 | 1.78 |
| GI | y = 2.746x0.405 | 0.44 | 0.64 | y = 1.496x1.718 | 0.67 | 1.67 |
| SPVI | y = 4.954x0.426 | 0.57 | 0.62 | y = 16.354x1.675 | 0.75 | 1.63 |
| NDVIcanste | y = 1.889e1.004x | 0.57 | 0.52 | y = 0.342e4.069x | 0.80 | 1.29 |
| NDRE | y = 4.968x0.438 | 0.56 | 0.52 | y = 17.04x1.758 | 0.77 | 1.42 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jin, X.; Yang, G.; Drummond, J.; Yang, H.; Clark, B.; Li, Z.; Zhao, C. Remote Sensing of Leaf and Canopy Nitrogen Status in Winter Wheat (Triticum aestivum L.) Based on N-PROSAIL Model. Remote Sens. 2018, 10, 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/rs10091463
Li Z, Jin X, Yang G, Drummond J, Yang H, Clark B, Li Z, Zhao C. Remote Sensing of Leaf and Canopy Nitrogen Status in Winter Wheat (Triticum aestivum L.) Based on N-PROSAIL Model. Remote Sensing. 2018; 10(9):1463. https://0-doi-org.brum.beds.ac.uk/10.3390/rs10091463
Chicago/Turabian StyleLi, Zhenhai, Xiuliang Jin, Guijun Yang, Jane Drummond, Hao Yang, Beth Clark, Zhenhong Li, and Chunjiang Zhao. 2018. "Remote Sensing of Leaf and Canopy Nitrogen Status in Winter Wheat (Triticum aestivum L.) Based on N-PROSAIL Model" Remote Sensing 10, no. 9: 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/rs10091463