Spatial Evolution of Prosopis Invasion and its Effects on LULC and Livelihoods in Baringo, Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Assessment of Land-Use and Land-Cover Changes
2.2.1. Field Reference Data
2.2.2. Satellite Data Selection and Pre-processing
2.2.3. Classification of Satellite Data
2.2.4. Accuracy Assessment
2.3. Land-Use and Land-Cover Change Analysis
3. Results
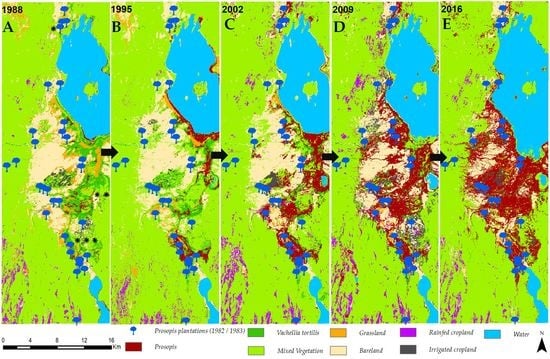
3.1. Invasion History of Prosopis from 1988 to 2016
3.2. Land-Use and Land-Cover Changes between 1988 and 2016
3.3. Prosopis-Specific Induced Changes on other LULC
4. Discussion
4.1. Spatial Evolution of Prosopis Invasion
4.2. Spatial Changes in Prosopis Coverage
4.3. LULC Changes in the Study Area
5. Conclusions
- Freely available Landsat data analyzed with the implementation of Random Forest machine learning algorithm in the open source R software are useful in assessing spatial temporal LULC changes, especially in regions where commercial data and software is economically inaccessible. The RF algorithm has the ability to separate various landscape components with reliable accuracies.
- The use of bi-seasonal (dry and wet) multispectral data combinations and the Random Forest algorithm allowed us to spatially and quantitatively investigate the evolution of Prosopis invasion, its current extent, and the changes in LULC in the semi-arid environment. The use of dry season images enhanced the ability to differentiate the evergreen Prosopis from deciduous native Vachellia species on the medium-resolution Landsat imagery.
- Prosopis has rapidly increased in the study area since its introduction in 1982. It currently stands at approximately 18,792 ha, invading at a rate of 640 ha per annum. This rapid spread was facilitated by effective seed dispersal agents such as livestock and wildlife, a lack of natural enemies, and favorable climate, among other factors. Our findings indicate that Prosopis has been a key driver of LULC changes in the semi-arid lowlands of Baringo, directly accounting for over a third of the LULC changes observed over the last three decades.
- The LULC classes most vulnerable to Prosopis invasion are grasslands, V. tortilis-dominated zones, and fallow irrigated agricultural fields. Their vulnerability is enhanced by their ecological niche also favoring Prosopis growth and the presence of Prosopis seed dispersal vectors such as livestock, wildlife, water, and humans.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Mooney, H.A. Invasive Alien Species: The Nature of the Problem; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- McGinley, M.; Duffy, J.E. Invasive Species. In Encyclopedia of Life; Encyclopedia of Earth: Washington, DC, USA, 2010. [Google Scholar]
- Brahic, C. Africa Trapped in Mega-Drought Cycle. NewScientist, 16 April 2009. Available online: https://www.newscientist.com/article/dn16967-africa-trapped-in-mega-drought-cycle/ (accessed on 28 January 2019).
- Ouden, J.; Mohren, G.M.J. Prosopis Upsurge in Kenya: Cause and Effects; Wageningen University & Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Gaulter, S. Analysis: Understanding the Sahel Drought. Scientists Say That the Current Drought in the Sahel Began as Far Back the 1960s; Al Jazeera Media Network: Doha, Qatar, 2012. [Google Scholar]
- Maghembe, A.J.; Kariuki, M.E.; Haller, D.R. Biomass and Nutrient Accumulation in Young Prosopis Juliflora at Mombasa, Kenya. Agrofor. Syst. 1983, 1, 313–321. [Google Scholar] [CrossRef]
- Herlocker, D.J.; Barrow, E.G.C.; Paetkau, P. A Preliminary Report on Trial Plantings of Woody Species in Arid and Semi-Arid Northern Kenya. In Proceedings of the Kenya National Seminar on Agroforestry; Buck, L., Ed.; [November, 1980]; ICRAF: Nairobi, Kenya, 1981; pp. 511–534. [Google Scholar]
- Pasiecznik, N.M.; Felker, P.; Harris, P.J.C.; Cruz, G.; Tewari, J.C.; Cadoret, K.; Maldonado, L.J. The Prosopis Julifora-Prosopis Pallida Complex: A Monograph; HDRA: Coventry, UK, 2001. [Google Scholar]
- Andersson, S. Spread of the Introduced Tree Species Prosopis Juliflora (Sw.) DC in the Lake Baringo Area, Kenya; Institutionen för Skoglig Vegetationsekologi Sveriges lantbruksuniversitet: UMEA, Sweden, 2005; pp. 1652–4918. [Google Scholar]
- Kaur, R.; Gonzáles, W.L.; Llambi, L.D.; Soriano, P.J.; Callaway, R.M.; Rout, M.E.; Gallaher, T.J.; Inderjit. Community Impacts of Prosopis Juliflora Invasion: Biogeographic and Congeneric Comparisons. PLoS ONE 2012, 7, e44966. [Google Scholar] [CrossRef]
- Kinyua, P.I.D. Agroforestry in Arid and Semi Arid Areas. In Agroforestry Development in Kenya: Proceedings of the Kenya National Seminar on Agroforestry, Held in Nairobi, Kenya, 7–16 November 1988; Kilewe, A.M., Kealey, K.M., Kebaara, K.K., Eds.; ICRAF: Nairobi, Kenya, 1989; pp. 442–450. [Google Scholar]
- Choge, S.K.; Pasiecznik, N.M. The Challenges of Eradicating Prosopis in Kenya; HDRA: London, UK, 2005. Available online: assets.publishing.service.gov.uk (accessed on 9 June 2016).
- Mwangi, E.; Swallow, B. Invasion of Prosopis Juliflora and Local Livelihoods: Case Study from the Lake Baringo Area of Kenya; World Agroforestry Center: Nairobi, Kenya, 2005. [Google Scholar]
- Brown, J.R.; Carter, J. Spatial and Temporal Patterns of Exotic Shrub Invasion in an Australian Tropical Grassland. Landsc. Ecol. 1998, 13, 93–102. [Google Scholar] [CrossRef]
- Shiferaw, H.; Schaffner, U.; Bewket, W.; Alamirew, T.; Zeleke, G.; Teketay, D.; Eckert, S. Modelling the Current Fractional Cover of an Invasive Alien Plant and Drivers of Its Invasion in a Dryland Ecosystem. Sci. Rep. 2019, 9, 1576. [Google Scholar] [CrossRef]
- Mirik, M.; Ansley, R. Comparison of Ground-Measured and Image-Classified Mesquite (Prosopis Glandulosa) Canopy Cover. Rangeland Ecol. Manag. 2012, 65, 85–95. [Google Scholar] [CrossRef]
- Adams, V.M.; Setterfield, S.A. Optimal Dynamic Control of Invasions: Applying a Systematic Conservation Approach. Ecol. Appl. 2015, 25, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Grice, A.C.; Clarkson, J.R.; Calvert, M. Geographic Differentiation of Management Objectives for Invasive Species: A Case Study of Hymenachne Amplexicaulis in Australia. Environ. Sci. Policy 2011, 14, 986–997. [Google Scholar] [CrossRef]
- Huang, C.; Asner, G.P. Applications of Remote Sensing to Alien Invasive Plant Studies. Sensors 2009, 9, 4869–4889. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.-T.; Rima, P.; Einzmann, K.; Immitzer, M.; Atzberger, C.; Eckert, S. Assessing the Potential of Sentinel-2 and Pléiades Data for the Detection of Prosopis and Vachellia Spp. in Kenya. Remote Sens. 2017, 9, 74. [Google Scholar] [CrossRef]
- Robinson, T.P.; Van Klinken, R.D.; Metternicht, G. Spatial and Temporal Rates and Patterns of Mesquite (Prosopis Species) Invasion in Western Australia. J. Arid Environ. 2008, 72, 175–188. [Google Scholar] [CrossRef]
- Adam, E.; Mureriwa, N.; Newete, S. Mapping Prosopis Glandulosa (Mesquite) in the Semi-Arid Environment of South Africa Using High-Resolution WorldView-2 Imagery and Machine Learning Classifiers. J. Aid Environ. 2017, 145, 43–51. [Google Scholar] [CrossRef]
- Van den Berg, E.C.; Kotze, I.; Beukes, H. Detection, Quantification and Monitoring of Prosopis in the Northern Cape Province of South Africa Using Remote Sensing and GIS. S. Afr. J. Geomat. 2013, 2, 68–81. [Google Scholar]
- Kyuma, R.K.; Wahome, R.G.; Kinama, J.M.; Wasonga, V.O. Temporal Relationship between Climate Variability, Prosopis Juliflora Invasion and Livestock Numbers in the Drylands of Magadi, Kenya. Afr. J. Environ. Sci. Technol. 2016, 10, 129–140. [Google Scholar] [CrossRef]
- Rembold, F.; Leonardi, U.; Ng, W.-T.; Gadain, H.; Meroni, M.; Atzberger, C. Mapping Areas Invaded by Prosopis Juliflora in Somaliland on Landsat 8 Imagery. In Proceedings of the SPIE Remote Sensing, Volume 9637, Remote Sensing for Agriculture, Ecosystems, and Hydrology XVII, Toulouse, France, 2015; p. 963723. [Google Scholar]
- Meroni, M.; Ng, W.-T.; Rembold, F.; Leonardi, U.; Atzberger, C.; Gadain, H.; Shaiye, M. Mapping Prosopis Juliflora in West Somaliland with Landsat 8 Satellite Imagery and Ground Information. Land Degrad. Dev. 2017, 28, 494–506. [Google Scholar] [CrossRef]
- Kigomo, J.N.; Luvanda, A.M.; Epodo, L. Experiences of Management and Utilization of Invasive Prosopis Juliflora in Turkana District. In Recent Advances in Forestry Research for Environmental Conservation, Improved Livelihood and Economic Development; Kenya Forestry Research Institute: Nairobi, Kenya, 2008; pp. 172–181. [Google Scholar]
- Choge, S.K.; Muthike, G. Experiences of Managing Prosopis Juliflora Invasions by Communities in Kenya: Challenges and Opportunities. In Proceedings of the Regional conference on Managing Prosopis Juliflora for better (agro-)pastoral Livelihoods in the Horn of Africa, Addis Ababa, Ethiopia, 1–2 May 2014; pp. 93–103. [Google Scholar]
- Kiage, K.-B.; Liu, K.B.; Walker, N.D.; Lam, N.; Huh, O.K. Recent Land-cover/Use Change Associated with Land Degradation in the Lake Baringo Catchment, Kenya, East Africa: Evidence from Landsat TM and ETM+. Int. J. Remote Sens. 2007, 28, 4285–4309. [Google Scholar] [CrossRef]
- Keitany, W.; Cherogony, E.; Riamangura, E.; Lentupuru, C.; Sadalla, L.; Kwonyike, A.; Bartenge, G.; Tomno, J.; Atuko, M.; Barno, J. First County Integrated Development Plan 2013–2017: Kenya Vision 2013, Towards a Globally Competitive and Prosperous Nation; County Government of Baringo: Kabarnet, Kenya, 2013. [Google Scholar]
- Olang, M.O. Classification of Kenya Rangeland. In Proceedings of the Third Workshop on African Forage Plant Genetic Resources, Evaluation of Forage Germplasm and Extensive Livestock Production Systems, Arusha, Tanzania, 27–30 April 1987; ILRI (aka ILCA and ILRAD): Nairobi, Kenya, 1988. [Google Scholar]
- Mwangi, E.; Swallow, B. Prosopis Juliflora Invasion and Rural Livelihoods in the Lake Baringo Area of Kenya. Conserv. Soc. 2008, 6, 130. [Google Scholar]
- Mwasi, B. Landscape Change Dynamics in a Semi-Arid Part of Baringo District, Kenya, Based on Landsat-TM Data and GIS Analysis; Universiteit van Amsterdam, IBED: Amsterdam, The Netherland, 2004. [Google Scholar]
- Wetang’ula, G.N.; Kubo, B.M.; Were, J.O. Environmental Baseline Study for Geothermal Developments: Case Study Arus-Bogoria Geothermal Prospects, Kenya. In Proceedings of the Short Course II on Surface Exploration for Geothermal Resources, Lake Naivasha, Kenya, 2–17 November 2007; Kenya Electricity Generating Company Ltd.: Lake Naivasha, Kenya, 2007. [Google Scholar]
- Kariuki, P. A Social Forestry Project in Baringo, Kenya: A Critical Analysis; University of Queensland: Brisbane, Australia, 1993. [Google Scholar]
- Getahum, A. Agroforestry for Development in Kenya. In Proceedings of the Kenya National Seminar on Agroforestry Agroforestry Developemnt in Kenya, Nairobi, Kenya, 7–16 November 1988; Kilewe, A.M., Kealey, K.M., Kebaara, K.K., Eds.; World Agroforestry (ICRAF): Nairobi, Kenya, 1989; pp. 85–109. [Google Scholar]
- Google Earth Pro. Google Earth Pro 7.3.2.5495. Baringo County, Kenya. 00 36′16.06″N, 35058′23.24″E. Viewed July 2016–December 2018. 2018 Google, US Dept. of State Geographer, Image archive for 1987–2016. Available online: https://softfamous.com/google-earth-pro/download/ (accessed on 20 December 2018).
- Masek, J.; Vermote, E.; Saleous, N.; Wolfe, R.; Hall, F.; Huemmrich, K.F.; Gao, F.; Kutler, J.; Lim, T.-K. A Landsat Surface Reflectance Data Set for North America, 1990–2000. IEEE Geosci. Remote Sens. Lett. 2006, 3, 68–72. [Google Scholar] [CrossRef]
- Roy, D.P.; Kovalskyy, V.; Zhang, H.K.; Vermote, E.F.; Yan, L.; Kumar, S.S.; Egorov, A. Characterization of Landsat-7 to Landsat-8 Reflective Wavelength and Normalized Difference Vegetation Index Continuity. Remote Sens. Environ. 2016, 185, 57–70. [Google Scholar] [CrossRef]
- Vidhya, R.; Duraisamy, V.; Ramakrishnan, S.S. Mapping Invasive Plant Prosopis Juliflora in Arid Land Using High Resolution Remote Sensing Data and Biophysical Parameters. Indian J. Geo-Mar. Sci. 2017, 46, 1135–1144. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. 2009; World Agroforestry (ICRAF): Nairobi, Kenya, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Package “RandomForest”: Breiman and Cutler’s Random Forests for Classification and Regression; cran.r-project, R Package Version 4-10: Vienna, Austria, 2014; Volume 4. [Google Scholar]
- Arlot, S.; Celisse, A. A Survey of Cross Validation Procedures for Model Selection. Stat. Surv. 2009, 4, 40–79. [Google Scholar] [CrossRef]
- Liaw, A.; Weiner, M. Random Forest: Breiman and Cutler’s Random Forests for Classification and Regression; cran.r-project, R Package Version 4.6-7: Vienna, Austria, 2012. [Google Scholar]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-Validation. In Encyclopedia of Database Systems; Liu, L., Özsu, M.T., Eds.; Springer: Boston, MA, USA, 2009; pp. 532–538. [Google Scholar]
- Maundu, P.; Kibet, S.; Morimoto, Y.; Imbumi, M.; Deka, R. Impacts of Prosopis Juliflora on Kenya’s Semi-Arid and Arid Ecosystems and Local Livelihoods. J. Life Earth 2009, 10, 17. [Google Scholar]
- Li, M.; Im, J.; Beier, C. Machine Learning Approaches for Forest Classification and Change Analysis Using Multi-Temporal Landsat TM Images over Huntington Wildlife Forest. GIScience Remote Sens. 2013, 50. [Google Scholar] [CrossRef]
- LI, Z.; Xin, X.; Tang, H.; Yang, F.; Chen, B.; Zhang, B. Estimating Grassland LAI Using the Random Forests Approach and Landsat Imagery in the Meadow Steppe of Hulunber, China. J. Integr. Agric. 2017, 16, 286–297. [Google Scholar] [CrossRef]
- Reynolds, J.; Wesson, K.; Desbiez, A.; Ochoa-Quintero, J.; Leimgruber, P. Using Remote Sensing and Random Forest to Assess the Conservation Status of Critical Cerrado Habitats in Mato Grosso Do Sul, Brazil. Land 2016, 5, 12. [Google Scholar] [CrossRef]
- Immitzer, M.; Böck, S.; Einzmann, K.; Vuolo, F.; Pinnel, N.; Wallner, A.; Atzberger, C. Fractional Cover Mapping of Spruce and Pine at 1 Ha Resolution Combining Very High and Medium Spatial Resolution Satellite Imagery. Remote Sens. Environ. 2018, 204, 690–703. [Google Scholar] [CrossRef]
- Relief Web. Baringo Residents Struggle with Drought and Conflict over Resources; ReliefWeb, UNOCHA: New York, NY, USA; Geneva, Switzerland, 2017. [Google Scholar]
- Wakie, T.T.; Laituri, M.; Evangelista, P.H. Assessing the Distribution and Impacts of Prosopis Juliflora through Participatory Approaches. Appl. Geogr. 2016, 66, 132–143. [Google Scholar] [CrossRef]
- Ndhlovu, T.; Milton, S.J.; Esler, K.J. Effect of Prosopis (Mesquite) Invasion and Clearing on Vegetation Cover in Semi-Arid Nama Karoo Rangeland, South Africa AU—Ndhlovu, Thabisisani. Afr. J. Range For. Sci. 2016, 33, 11–19. [Google Scholar] [CrossRef]
- Henderson, L. Invasive, Naturalized and Casual Alien Plants in Southern Africa: A Sum mary Based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 2007, 37, 215–248. [Google Scholar] [CrossRef]
- Babiker, A.G.T. Mesquite (Prosopis Spp.) in Sudan: History, Distribution and Control. Available online: WordPress.com (accessed on 27 January 2018).
- Muturi, G.M.; Mohren, G.M.J.; Kimani, J.N. Prediction of Prosopis Species Invasion in Kenya Using Geographical Information System Techniques. Afr. J. Ecol. 2009, 48, 628–636. [Google Scholar] [CrossRef]
- Kimosop, W.; Kidundo, M.; Musila, F.; Cheruiyot, M.; Kangogo, M.; Ngeche, K.; Chepkonga, S.; Koros, D.; Meto, J.; Harper, D. Lake Bogoria National Reserve: Integrated Management Plan 2007–2012; 2007 County Council of Baringo, County Council of Koibatek and WWF-EARPO: Baringo, Kenya, 2007. [Google Scholar]
- Muthana, K.D.; Arora, G.D. Prosopis Juliflora (Swartz) D.C., a Fast Growing Tree to Bloom the Desert. Director, Central Arid Zone Research Institute, printed at Rajasthan Law Weekly Press, Jodhpur—342 003, INDIA, CAZRI Monograph No. 22. December 1983. [Google Scholar]
- Dubow, A.Z. Mapping and Managing the Spread of Prosopis Juliflora in Garissa County, Kenya; Kenyatta University: Nairobi City, Kenya, 2005. [Google Scholar]
- Villagra, P.; Vilela, A.; Giordano, C.; Alvarez, J. Ecophysiology of Prosopis Species from the Arid Lands of Argentina: What Do We Know About Adaptation to Stressful Environments? In Desert Plants Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 321–340, pp. 321–340. [Google Scholar]
- Schachtschneider, K.; February, E.C. Impact of Prosopis Invasion on a Keystone Tree Species in the Kalahari Desert. Plant Ecol. 2013, 214, 597–605. [Google Scholar] [CrossRef]
- WIKIPEDIA. Perkerra River. Available online: wikipedia.org (accessed on 8 February 2019).
- KALRO, K. Rainfall Data for Marigat for 39 Years _1970–2008; KALRO: Marigat, Kenya, 2016. [Google Scholar]
- Nicholson, S.E.; Funk, C.; Fink, A.H. Rainfall over the African Continent from the 19th through the 21st Century. Glob. Planet. Chang. 2018, 165, 114–127. [Google Scholar] [CrossRef]
- Daily Nation. Why Water Levels Are Rising in Kenya’s Rift Valley Lakes. Kenya. Available online: www.nation.co.ke (accessed on 15 April 2019).
- Choge, S.K.; KEFRI, Baringo Sub-Centre, Marigat, Kenya. Irrigation Activities in Marigat. Personal communication, 18 September 2018. [Google Scholar]
- Ng’etich, P. Seed Firms’ Offer of Life for Famished Baringo. 2008. Available online: www.nation.co.ke (accessed on 19 February 2019).
- Kilawe, C.J.; Mbwambo, J.R.; Kajembe, G.C.; Mwakalukwa, E.E. Mrashia: Prosopis Has Started Invading Pastures and Agricultural Lands in Tanzania. The Woody Weeds Project. 2017. Available online: www.woodyweeds.org (accessed on 6 February 2019).
- Choge, S.K.; KEFRI, Baringo Sub-Centre, Marigat, Kenya; Lekruito, J.; CPA Chairman, Ngambo Sublocation, Marigat, Kenya. Ban on Vachellia Utilization in Marigat. Personal communication, 21 June 2018. [Google Scholar]
- Kenya Law. CHARLES LEKUYEN NABORI & 9 OTHERS v ATTORNEY GENERAL & 3 OTHERS [2007] EKLR, Petition 466 of 2006. 2007. Available online: kenyalaw.org (accessed on 20 February 2019).
- Lugusa, K. Fodder Production as an Adaptation Strategy in the Drylands: A Case Study of Producer Groups in Baringo County, Kenya; University of Nairobi Research Archive: Nairobi, Kenya, 2015. [Google Scholar] [CrossRef]
- Goel, V.L.; Behl, H.M. Growth, biomass estimations and fuel quality evaluation of coppice plants of Prosopis Juliflora on sodic soil site. J. Trop. For. Sci. 2000, 12, 139–148. [Google Scholar]
- Shackleton, R.T.; Le Maitre, D.C.; Pasiecznik, N.M.; Richardson, D.M. Prosopis: A Global Assessment of the Biogeography, Benefits, Impacts and Management of One of the World’s Worst Woody Invasive Plant Taxa. AoB Plants 2014, 6, plu027. [Google Scholar] [CrossRef]
- Van Klinken, R.D.; Campbell, S.D. The Biology of Australian Weeds 3 Prosopis L. Species. Plant Prot. Q. 2001, 16, 1–19. [Google Scholar]
- Muturi, G.M.; Poorter, L.; Mohren, G.M.J.; Kigomo, B.N. Ecological Impact of Prosopis Species Invasion in Turkwel Riverine Forest, Kenya. J. Arid Environ. 2013, 92, 89–97. [Google Scholar] [CrossRef]
- Ayanu, Y.; Jentsch, A.; Müller-Mahn, D.; Rettberg, S.; Romankiewicz, C.; Koellner, T. Ecosystem Engineer Unleashed: Prosopis Juliflora Threatening Ecosystem Services? Reg. Environ. Chang. 2015, 15, 155–167. [Google Scholar] [CrossRef]
- Dahl, E.B.; Sosebee, R.; Goen, J.P.; Brumley, C.S. Will Mesquite Control with 2,4,5-T Enhance Grass Production? J. Range Manag. 1978, 31, 129. [Google Scholar] [CrossRef]
- Ntshidi, Z.; Dzikiti, S.; Mazvimavi, D.; Bugan, R.; Le Maitre, D.; Gush, M.; Jovanovic, N. Comparative use of groundwater by invasive alien Prosopis spp. and co-occurring indigenous v. Karroo in a semi-arid catchment. In Proceedings of the 14th Biennial Ground Water Division Conference and Exhibition, Muldersdrift, South Africa, 21–23 September 2015. [Google Scholar]
- Dzikiti, S.; Schachtschneider, K.; Naiken, V.; Gush, M.; Moses, G.; Le Maitre, D.C. Water Relations and the Effects of Clearing Invasive Prosopis Trees on Groundwater in an Arid Environment in the Northern Cape, South Africa. J. Arid Environ. 2013, 90, 103–113. [Google Scholar] [CrossRef]
- Dzikiti, S.; Ntshidi, Z.; Le Maitre, D.C.; Bugan, R.D.H.; Mazvimavi, D.; Schachtschneider, K.; Jovanovic, N.Z.; Pienaar, H.H. Assessing Water Use by Prosopis Invasions and Vachellia Karroo Trees: Implications for Groundwater Recovery Following Alien Plant Removal in an Arid Catchment in South Africa. For. Ecol. Manag. 2017, 398, 153–163. [Google Scholar] [CrossRef]
- Fourie, F.; Mbatha, K.; Verster, H.; Dyk, G.V. The Effect of Vegetation (Prosopis Sp.) on Groundwater Levels in Rugseer River, Kenhardt, South Africa. Available online: http://www.dwaf.gov.za (accessed on 4 April 2019).







| Class ID | LULC | Classification Description |
|---|---|---|
| 1 | Prosopis | Refers to P. juliflora, which is an evergreen woody alien shrub predominating the lowland areas around Lake Baringo |
| 2 | Vachellia tortilis | An indigenous tree species predominantly found within the tropical arid and semi-arid lands (ASALs) |
| 3 | Mixed Vegetation | A combination of natural trees and shrubs that exist as a mix of single stands or as small patches and water weeds |
| 4 | Grassland | Areas predominantly under grass cover |
| 5 | Bareland | Degraded areas usually with little or no vegetation (uncovered soils) |
| 6 | Rainfed cropland | Areas for farming that depend on rainfall for water |
| 7 | Irrigated cropland | Areas for farming equipped to provide water |
| 8 | Water | Rivers, lakes, and dams |
| Sensor | Acquisition Date | Year Assigned to Classification | |
|---|---|---|---|
| Dry Season | Wet Season | ||
| Landsat 5 TM | Mar, 1989 | July, 1987 | 1988 |
| Landsat 5 TM | Jan, 1995 | Mar,1995 | 1995 |
| Landsat 5 TM | Feb, 2002 | July, 2002 | 2002 |
| Landsat 5 TM | Jan, 2010 | June, 2008 | 2009 |
| Landsat 8 OLI | Feb, 2016 | July, 2015 | 2016 |
| LULC Classes | 1988 | 1995 | 2002 | 2009 | 2016 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ha | % Share | ha | % Share | ha | % Share | ha | % Share | ha | % Share | |
| Prosopis | 882 | 0.5 | 3345 | 1.9 | 8375 | 4.7 | 13,568 | 7.5 | 18,792 | 10.4 |
| Vachellia tortilis | 8517 | 4.7 | 6809 | 3.8 | 3158 | 1.8 | 3718 | 2.1 | 4915 | 2.7 |
| Mixed vegetation | 128,727 | 71.5 | 130,385 | 72.4 | 132,969 | 73.9 | 124,392 | 69.1 | 123,310 | 68.5 |
| Grassland | 7229 | 4.0 | 5652 | 3.1 | 1194 | 0.7 | 691 | 0.4 | 977 | 0.5 |
| Bareland | 15,001 | 8.3 | 16,904 | 9.4 | 14,130 | 7.9 | 13,420 | 7.5 | 8503 | 4.7 |
| Rainfed cropland | 3840 | 2.1 | 3189 | 1.8 | 5531 | 3.1 | 5453 | 3.0 | 2408 | 1.3 |
| Irrigated cropland | 1501 | 0.8 | 473 | 0.3 | 1463 | 0.8 | 3708 | 2.1 | 652 | 0.4 |
| Water | 14,325 | 8.0 | 13,264 | 7.4 | 13,204 | 7.3 | 15,071 | 8.4 | 20,464 | 11.4 |
| Net Changes | 1988–1995 | 1995–2002 | 2002–2009 | 2009–2016 | 1988–2016 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LULC | ha | % Total Area | % of Class Area in 1988 | ha | % Total Area | % of Class Area in 1988 | ha | % Total Area | % of Class Area in 1988 | ha | % Total Area | % of Class Area in 1988 | ha | % Total Area | % of Class Area in 1988 |
| Prosopis | 2463 | 1.4 | 279.4 | 5030 | 2.8 | 570.4 | 5193 | 2.9 | 589.0 | 5224 | 2.9 | 592.5 | 17910 | 10.0 | 2031.3 |
| Vachellia tortilis | −1708 | −1.0 | −20.1 | −3651 | −2.0 | −42.9 | 560 | 0.3 | 6.6 | 1197 | 0.7 | 14.1 | −3602 | −2.0 | −42.3 |
| Mixed vegetation | 1658 | 0.9 | 1.3 | 2583 | 1.4 | 2.0 | −8576 | −4.8 | −6.7 | −1082 | −0.6 | −0.8 | −5417 | −3.0 | −4.2 |
| Grassland | −1577 | −0.9 | −21.8 | −4458 | −2.5 | −61.7 | −503 | −0.3 | −7.0 | 286 | 0.2 | 4.0 | −6252 | −3.5 | −86.5 |
| Bareland | 1903 | 1.1 | 12.7 | −2774 | −1.5 | −18.5 | −709 | −0.4 | −4.7 | −4917 | −2.7 | −32.8 | −6498 | −3.6 | −43.3 |
| Rainfed cropland | −651 | −0.4 | −17.0 | 2342 | 1.3 | 61.0 | −78 | −0.0 | −2.0 | −3045 | −1.7 | −79.3 | −1432 | −0.8 | −37.3 |
| Irrigated cropland | −1027 | −0.6 | −68.5 | 989 | 0.6 | 66.0 | 2246 | 1.3 | 149.7 | −3056 | −1.7 | −203.7 | −849 | −0.5 | −56.6 |
| Water | −1061 | −0.6 | −7.4 | −61 | −0.0 | −0.4 | 1868 | 1.0 | 13.0 | 5393 | 3.0 | 37.6 | 6139 | 3.4 | 42.9 |
| Time Period | Losses to P (ha) | Gains from P (ha) | Net Change (ha) | % of Class Area in 1988 |
|---|---|---|---|---|
| V88-P95 | 984 | 235 | −750 | −8.8 |
| V95-P02 | 2146 | 237 | −1909 | −22.4 |
| V02-P09 | 789 | 409 | −381 | −4.5 |
| V09-P16 | 913 | 281 | −633 | −7.4 |
| V88-P16 | 3478 | 26 | −3453 | −40.5 |
| M88-P95 | 651 | 227 | −424 | −0.3 |
| M95-P02 | 2183 | 725 | −1458 | −1.1 |
| M02-P09 | 3837 | 1340 | −2498 | −1.9 |
| M09-P16 | 4247 | 1753 | −2494 | −1.9 |
| M88-P16 | 6308 | 93 | −6215 | −4.8 |
| G88-P95 | 510 | 82 | −428 | −5.9 |
| G95-P02 | 1042 | 163 | −879 | −12.2 |
| G02-P09 | 341 | 170 | −171 | −2.4 |
| G09-P16 | 126 | 285 | 159 | 2.2 |
| G88-P16 | 2688 | 13 | −2675 | −37 |
| B88-P95 | 50 | 5 | −44 | −0.3 |
| B95-P02 | 1009 | 124 | −885 | −5.9 |
| B02-P09 | 2977 | 856 | −2121 | −14.1 |
| B09-P16 | 3644 | 974 | −2670 | −17.8 |
| B88-P16 | 5361 | 10 | −5351 | −35.7 |
| R88-P95 | 17 | 0.7 | −17 | −0.4 |
| R95-P02 | 5 | 16 | 11 | 0.3 |
| R02-P09 | 709 | 139 | −571 | −14.9 |
| R09-P16 | 423 | 136 | −287 | −7.5 |
| R88-P16 | 131 | 3 | −129 | −3.4 |
| I88-P95 | 44 | 0 | −44 | −2.9 |
| I95-P02 | 7 | 58 | 51 | 3.4 |
| I02-P09 | 456 | 368 | −89 | −5.9 |
| I09-P16 | 1287 | 173 | −1115 | −74.3 |
| I88-P16 | 378 | 5 | −373 | −24.9 |
| W88-P95 | 758 | 1 | −757 | −5.3 |
| W95-P02 | 44 | 84 | 40 | 0.3 |
| W02-P09 | 0.1 | 637 | 636 | 4.4 |
| W09-P16 | 7 | 1822 | 1814 | 12.7 |
| W88-P16 | 10 | 295 | 285 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbaabu, P.R.; Ng, W.-T.; Schaffner, U.; Gichaba, M.; Olago, D.; Choge, S.; Oriaso, S.; Eckert, S. Spatial Evolution of Prosopis Invasion and its Effects on LULC and Livelihoods in Baringo, Kenya. Remote Sens. 2019, 11, 1217. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11101217
Mbaabu PR, Ng W-T, Schaffner U, Gichaba M, Olago D, Choge S, Oriaso S, Eckert S. Spatial Evolution of Prosopis Invasion and its Effects on LULC and Livelihoods in Baringo, Kenya. Remote Sensing. 2019; 11(10):1217. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11101217
Chicago/Turabian StyleMbaabu, Purity Rima, Wai-Tim Ng, Urs Schaffner, Maina Gichaba, Daniel Olago, Simon Choge, Silas Oriaso, and Sandra Eckert. 2019. "Spatial Evolution of Prosopis Invasion and its Effects on LULC and Livelihoods in Baringo, Kenya" Remote Sensing 11, no. 10: 1217. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11101217