1. Introduction
As one of the world’s major food crops, wheat has a huge market demand in China [
1], and its production status directly affects the country’s stability of agricultural product. The demand for high-quality food products has increased in recent decades in China [
2] and grain protein content (GPC) is an important quality index for wheat [
3]. Protein content above 12.5% in wheat provides sufficient gluten to form good dough for bread making, while wheat with protein content under 11% is suitable for making cookies. GPC is the main measurement of wheat quality and it is determined by the genetic background and, to a large extent, environmental factors, such as N supply, as well as water and temperature conditions [
4,
5,
6,
7,
8]. Advanced site-specific knowledge of GPC will provide opportunities to adopt optimized strategies for grain harvesting [
3]. Therefore, real-time monitoring of plant N status and a pre-harvest prediction of the grain and/or protein yield in wheat can assist producers in improving N management strategies, as well as in generating yield and quality maps [
4].
The formation of grain protein is physically dependent on plant nitrogen accumulation and its translocation from leaves and shoots to the grains in the grain filling stage [
3,
9,
10,
11,
12,
13,
14]. Most of the nitrogen that is converted into protein is taken up prior to anthesis, stored in the leaves, and remobilized during grain fill [
15]. The plant may take up nitrogen both pre- and post-anthesis [
14]. The greatest part of the nitrogen present in the harvest is assimilated pre-anthesis in the aboveground parts and is mobilized in the vegetative parts and redistributed to the grains [
9,
10,
11,
12,
13,
14]. Wang et al. [
16] showed that the nitrogen content of winter wheat at the anthesis stage was indicative for the final grain protein content and the correlation coefficient between the leaf nitrogen concentration at anthesis and the grain protein content was 0.726 (
n = 26). The nitrogen in the leaves is an important component of chlorophyll and the enzymes involved in photosynthesis [
15]. Leaf nitrogen content (LNC) is an important indicator of the crop photosynthetic capacity [
17] and is needed by agronomists for fertilizer recommendations [
18]. Quantification of LNC can provide valuable information for monitoring crop physiology [
19] and practicing precision farming [
20] so as to improve the use and efficiency of nitrogen fertilizers.
Remote sensing has been widely used as a non-destructive approach for estimating the leaf N content in the past few decades [
21,
22,
23,
24]. Leaf N accumulation (LNA) can provide comprehensive information about leaf dry matter and LNC, thus reflecting leaf N status, as well as vegetation coverage during crop growth [
25]. Meanwhile, plant N concentration (PNC) and accumulation (PNA) have also been used as indicators for assessing the plant N status for crops [
26]. PNC, expressed on a land area basis, is the product of the plant N concentration and dry biomass. It can be used to indicate the N status of crops in the same growth stage [
27]. PNA is highly variable within a single year and between years, sites, and crops, even when the N supplies from both the soil and additional fertilizer inputs are plentiful [
28].
There are many studies regarding the estimation of the plant nitrogen status from canopy spectral reflectance data [
29,
30]. It is possible to predict GPC from remote sensing data if the remote sensing model of the plant nitrogen content is integrated with an agronomic model of the grain protein based on the plant nitrogen accumulation at the wheat anthesis stage [
3]. Wang et al. [
31] obtained a good inversion effect by constructing a GPC prediction model based on wheat canopy spectral parameters and LNC. Xue et al. [
4] showed a strong relationship between the leaf N status and GPC, which indicates that canopy spectra can be used to predict GPC [
32]. Huang et al. [
33] found that the ratio of carotenoids to chlorophyll a in winter wheat leaves can be used as an intermediate variable to establish an inversion model between the spectral characteristics of wheat and the GPC.
As a means of rapid, non-destructive, and large-area simultaneous monitoring, satellite remote sensing technology has been proved to be useful for the inversion of various physiological and biochemical parameters of crops [
34,
35]. It is possible to use the remote sensing information to invert crop physiological and biochemical parameters and to monitor crop quality [
36].
Satellite remote sensing enables growers to obtain spatially explicit information about crop conditions to make both within-season management decisions and post-season evaluations relating to nutrient or irrigation management zones [
37]. The Landsat Thematic Mapper (TM) is the most commonly used satellite platform for assessing the spatial variability of crop conditions, including biomass, leaf area, gross primary production, and yield [
32,
38,
39,
40]. Eitel et al. [
41,
42] simulated the broad-bands used by RapidEye from ground-based hyperspectral reflectance data to disentangle the contributions of wheat biochemistry (Chl, N) and structure (leaf area) in the prediction of N concentration. Perry et al. [
43] compared measurements of ground-based vegetation indices (Vis) sensitive to N concentration (i.e., CCCI) using RapidEye imagery and found that the prediction of N concentration from satellites was confounded by inaccurate measurements of biomass and by challenges associated with scaling ground measurements to satellite pixels [
32]. Liu et al. [
44] constructed an inversion model between the vegetation index and winter wheat GPC using remote sensing images of the wheat anthesis and filling stages and obtained satisfactory inversion accuracy. Reyniers et al. [
45] calculated the normalized vegetation index (NDVI) through the spectral parameters obtained by color infrared aerial images collected before wheat harvest and the Cropscan Spectrometer and established a model for predicting GPC quality. Li et al. (2012) established the GPC estimation model with multi-temporal Landsat TM/ETM data through a generalized regression neural network (GRNN) method [
46].
Sentinel-2 is the latest generation of Earth observation satellites launched by the European Space Agency (ESA) in recent years. The Sentinel-2 mission consists of two satellites developed to support vegetation, land cover, and environmental monitoring. The Sentinel-2A satellite was launched by the ESA on 23 June 2015 and operates in a sun-synchronous orbit with a 10-day repeat cycle. A second identical satellite (Sentinel-2B) was launched on 7 March 2017. The Sentinel-2 MultiSpectral Instrument (MSI) acquires 13 spectral bands ranging from visible and near-infrared (VNIR) to shortwave-infrared (SWIR) wavelengths along a 290-km orbital swath. The spatial resolution of the four bands of blue, green, red, and near-infrared is 10 meters. Among the multispectral optical satellite data, Sentinel 2A/2B data is the only available data with three bands in the red-edge range, which is very effective for detecting vegetation information. Clevers et al. [
47] have proved that these three red-edge bands are particularly suitable for estimating canopy chlorophyll and nitrogen (N) content.
The aim of this work is to investigate whether using VIs derived from Sentinel-2A/2B (with an emphasis on the red-edge band) can be used to detect the wheat N status and, furthermore, to quantitatively forecast the wheat grain protein of crops before they fully ripen. The objectives of this study are: (1) To determine the predictive capability of commonly used VIs collected during wheat “flowering/anthesis” stages to estimate N status in wheat using simulated Sentinel-2 broad-bands VIs from ground-based hyperspectral data; and (2) to evaluate the performance of a wheat GPC detection model based on different N parameters (PNA, PNC, LNA, and LNC) across a range of years, farms, and growing conditions and provide county-scale maps of wheat N parameters and GPC distribution for anthesis seasons (2017–2018) and to examine the model’s precision.
2. Materials and Methods
2.1. Experimental Design
This study was conducted across three location representatives of the middle-high precipitation zone (600 mm annually) in the rainfed wheat-maize rotation region of Northern China. The 2003–2006 winter wheat experiments were carried out in the suburbs of Beijing; the 2013–2015 experiments were carried out in the National Experimental Station for Precision Agriculture in Beijing; and the 2017–2018 experiment was carried out in Renqiu, Hebei Province, south of Beijing (
Figure 1). The summary for experiments and data collected in this study are listed in
Table 1.
The climate characteristics of our study area are a typical warm temperate semi-humid monsoon continental climate, with high temperatures and rain in summer, cold and dry in winter, and short duration in spring and autumn. The annual frost-free stage is 180–200 days and the annual average temperature is 8–12 °C. The annual average precipitation spatial distribution is uneven (from 500 to 700 mm) and the rainfall season distribution is also very uneven as the summer precipitation accounts for about 80% of the annual precipitation and is mainly concentrated in June, July, and August. The average annual sunshine hours are between 2000 and 2800 h.
The National Experimental Station for Precision Agriculture is located in Xiaotangshan Town, Changping District, Beijing (40°10′33″ N–40°11′20″ N, 116°26′10″ E–116°27′06″ E). Trial 1, Trial 2, and Trial 3 were conducted in this station during 2013–2015.
Trial 1: The 2012–2013 trial was a randomized block with two replicates. The nitrogen treatments in the 2012–2013 growing season were 0 kg N per hectare, 104 kg N per hectare, 208 kg N per hectare, and 416 kg N per hectare. Nongda 211, Zhongmai 175, Zhongyou 206, and Jing 9843 were chosen as the test cultivars. The plot area was 90 m2 (10 m × 9 m). The wheat sowing date was 28 September 2012. In addition to the nitrogen applied to each treatment, the fertilizer level was 60 kg/ha P2O5 and 76.5 kg/ha K2O, and the irrigation amount was 187 mm. The data obtained from this experiment were used for crop N status and GPC modeling.
Trial 2: The 2013–2014 trial was an orthogonal experimental design, with three factors including N rates, varieties, and irrigation volume over 2013–2014. The winter wheat varieties were Jing 9843 and Zhongmai 175. Four nitrogen levels (0, 90, 180, and 360 kg N per hectare) were used. The irrigation volume levels were 25, 171, and 317 mm. The plot area was 150 m2 (10 m × 15 m) with three replicates for a total of 48 cells. The sowing date was 4 October 2013. In addition to the nitrogen applied to each treatment, the fertilizer level was 60 kg/ha P2O5 and 76.5 kg/ha K2O, and the irrigation amount was 187 mm. The other field management parameters were the same as the local standard practices. The data obtained from this experiment was used for crop N status and GPC modeling.
Trial 3: At 2014–2015 trial, two winter wheat varieties, J9843 and ZM175 were selected for ground measurements during the flowering periods of 2015. Winter wheat was grown on 48 plots and four amounts of nitrogen fertilizer (N0 is no fertilizer, N1 is 195 kg/ha, N2 is 390 kg/ha, and N3 is 780 kg/ha) and three irrigation levels (W0 is rainfall only, W1 is rainfall plus 100 mm, and W2 is rainfall plus 200 mm) were applied in this trail. The data obtained from this experiment was used to evaluate the scale effects for winter N status estimation modeling used in this study.
Trial 4 to Trial 7 were conducted in Beijing suburbs from 2003 to 2006. The experiments were carried out in Tongzhou, Daxing, Fangshan, Shunyi, and Changping counties of Beijing. For each year, 10–30 wheat farmlands were selected as study fields. The geographical locations for these fields ranged from 115°25′ to 117°30′ in the east longitude and 39°38′ to 40°51′ in the north latitude (
Figure 1). The farmlands selected in this study were flat and uniform in wheat growth, the area for each field was more than 5 ha. The farmlands were cultivated by farmers and managed by uniform fertilizer and water without special treatment. Remote sensing monitoring experiments were conducted at the wheat anthesis stage (17 May 2003; 18 May 2004; 8 May 2005; 10 May 2006). During each field trial, winter wheat in the middle of the field was selected as the sampling subplot. Plant samples were taken almost synchronously with the spectral measurements. Plant samples were immediately sealed in plastic bags and transported to the laboratory for subsequent analysis. All sampling locations were positioned using a handheld GPS. The data obtained from these experiments were used for model validation.
The 2017–2018 trial was carried out in the Renqiu area, which is located in the Cangzhou city of Hebei Province (38°32′17″ N–38°50′50″ N, 115°55′59″ E–116°22′55″ E). During the wheat anthesis stage in 2018, from 5 May to 10 May, 20 wheat fields whose area was greater than 10 ha were selected in the Renqiu area for ground investigation. In the middle of each trial field, plants with uniform growth were taken for four rows with length of 60 cm. All plant samples were uprooted and placed in the sample bag, sealed, and sent to the laboratory for further processing. All sampling positions were located with a handheld GPS.
2.2. Experimental Data Acquisition
2.2.1. Canopy Spectral Measurement
Determination of the canopy spectra of wheat was carried out during the wheat anthesis stage (17 May 2003; 18 May 2004; 8 May 2005; 10 May 2006; 22 May 2013; 7 May 2014). The ground hyperspectral uses the Fieldspec FR2500 field spectral emission spectrometer (ASD, Boulder, CO, USA) with a spectral range of 350–2500 nm, a spectral resolution of 1.4 nm at 350–1000 nm and 2 nm at 1000–2500 nm, and the spectral resampling interval is 1 nm. When measuring, the probe is measured perpendicular to the top of the canopy about 1.3 m. Calibration was performed before and after the measurement using a calibration plate. Each cell was measured 20 times when the weather was sunny and cloudless between 10:00 and 14:00, and the average was taken as the canopy spectrum of the treatment. In this study, the wheat canopy hyperspectral data from Trial 1 to 2, Trial 4 to 7 was all convolved to the spectral band configuration of Sentinel-2 using the expected spectral response function of Sentinel-2 [
48] through ENVI (The Environment for Visualizing Images) software, in order to evaluate the spectral response of wheat nitrogen in each Sentinel-2 channel.
2.2.2. Winter Wheat LAI and AGB Data
After canopy spectral measurements were completed, samples were collected for the determination of wheat leaf area index (LAI), wheat leaves, stems, ears, as well as wheat above ground biomass (AGB). In this study, wheat plants from a 40 × 50 cm subplot within each field were cut with scissors, then placed in a plastic bag and transported to the laboratory for subsequent analysis. All leaves, stems, and ears from 20 wheat plants in each plot were removed, put into a paper bag and dried at 80 °C to remove moisture. Once the sample weight became constant (about 24 h), they were weighed using a balance accurate to 0.001 g. Finally, the biomass per unit area was calculated based on the measured planting density and the dry weight of the samples. Winter wheat LAI and above ground biomass (AGB) for leaves (LAGB), stems (SAGB), and ears (EAGB) was calculated using:
where
Sa is the total area of all the leaves for 20 wheat plants,
is the number of winter wheat plants per unit area,
is the number of selected winter wheat plants (
p = 20 in this study),
is the dry weight of the wheat leaf sample for 20 wheat plants,
is the dry weight of the wheat stem sample for 20 wheat plants,
is the dry weight of the wheat ear sample for 20 wheat plants.
2.2.3. Winter Wheat Nitrogen Parameters
After measurement of the AGB, the leaf, stem, and ear samples were separated, ground, and passed through a 40-mesh screen. Wheat leaf nitrogen content (LNC), stem nitrogen content (SNC), and ear nitrogen content (ENC) were determined using a Kjeldahl nitrogen analyzer B-339 (Buchi AG, Flawil, Switzerland). Then three other wheat nitrogen parameters, LNA, PNC, and PNA, which represent wheat leaf- and canopy-level nitrogen status, were calculated using Equations (6) to (8):
2.2.4. Winter Wheat GPC Data
For experiments conducted at the National Experimental Station for Precision Agriculture during 2012–2014, after the wheat matured, 1 m2 of wheat was taken from each cell, dried, and threshed. Winter wheat’s GPC was determined using an Infratec TM 1241 Near-Infrared Grain Analyzer (FOSS Inc., Denmark).
For experiments conducted in the Beijing suburbs during 2003–2006 and in Renqiu during 2017–2018, five representative 1-m2 areas of wheat plant were taken from each field by hand after the wheat matured, and then the wheat grain was dried and threshed. The winter wheat’s GPC was determined by an Infratec TM 1241 near-infrared grain analyzer.
2.2.5. Acquisition and Processing of Unmanned Aerial Vehicle (UAV) Remote-Sensing Images
An UAV sensor platform, DJI S1000 UAV (SZ DJI Technology Co., Ltd., Sham Chun, China) with eight propellers, which is very stable at low flight speed and low altitude, was used in Trail 3 in this study. The UHD 185 Firefly (UHD 185 firefly, Cubert GmbH, Ulm, Baden-Württemberg, Germany) is a snapshot hyperspectral sensor. The UHD 185 has a short exposure and integration time, weighs 0.47 kg, and measures 195 × 67 × 60 mm
3. Its operating range spans from the visible to the near-infrared (wavelength range: 450 nm to 950 nm, 8 nm @ 532 nm). Hyperspectral data cubes were automatically resampled to 4 nm spacing. Collected radiation is recorded as a 1000 × 1000 (1 band) panchromatic image and a 50 × 50 (125 bands) hyperspectral cube. The fusion steps are implemented in Cubert Cube-Pilot software (Cube-Pilot, Version 1.4, Cubert GmbH, Ulm, Baden-Württemberg, Germany). After fusion, all hyperspectral images with 1000 × 1000 (125 bands) were stitched together using an image stitching process, and the final result is shown in
Figure 2.
Flights were conducted during the wheat anthesis stage (13 May 2015). The UAV flight altitude is 50 m and the spatial resolution of UAV-UHD image is 0.01 m. The UHD 185 was calibrated on the ground before the UAV flight by using Cubert Cube-Pilot software (Version 1.4) and a BaSO4 whiteboard. The original DN values of the UAV-DC images were calibrated by imaging a black-and-white fabric placed on the ground and using
where
is the band names, such as R, G, B,
is the original DN value of the high-definition digital camera images; and
and
are the original DN values from the white-and-black fabric.
In this study, the UAV hyperspectral image from Trial 3 was first convolved to the spectral band configuration of Sentinel-2 using the expected spectral response function of Sentinel-2 [
48] through ENVI software. Then, the original 0.02 m simulated sentinel-2 image was resized to 0.5 m to 2.5 m spatial resolution images using the ENVI Resize tool in this study in order to estimate the wheat N and GPC estimation model accuracy.
2.2.6. Sentinel-2 Satellite Data Acquisition and Preprocessing
A total of seven scenes of Sentinel-2A/2B images were obtained in this experiment (
Table 3). The radiometric calibration and atmospheric correction of Sentinel-2A/2B in this study was completed using the plugin Sen2Cor-02.05.05 in the SNAP software. Sentinel band 5 to band 7, band 11, and band 12 data were resampled to 10-m spatial resolution using the SNAP software and then exported to the ENVI format. The follow-up work, such as wheat area extraction and VIs calculation, were all completed in ENVI5.3 [
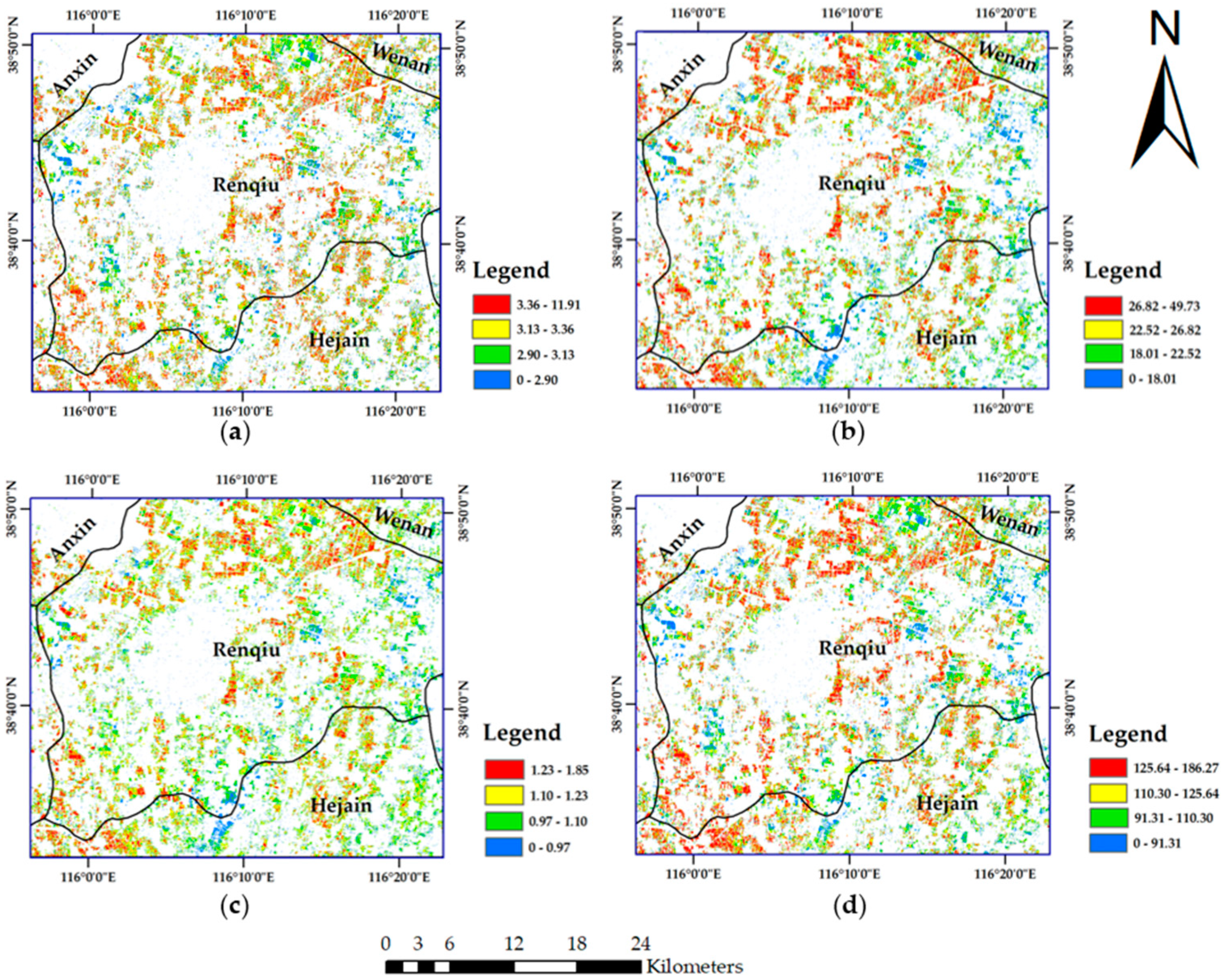
49]. The Sentinel-2B image of 8 May 2018, which was collected in the wheat anthesis season, was used as the data source for wheat nitrogen monitoring and GPC prediction. The other six scenes, which cover the winter wheat greening stage to the harvest stage in the Renqiu area, were used to extract the winter wheat planting area in 2018.
A supervised classification method of maximum likelihood combined with decision tree classification was used to complete the extraction of the winter wheat planting area. The wheat extraction results are shown in
Figure 3.
2.3. Method
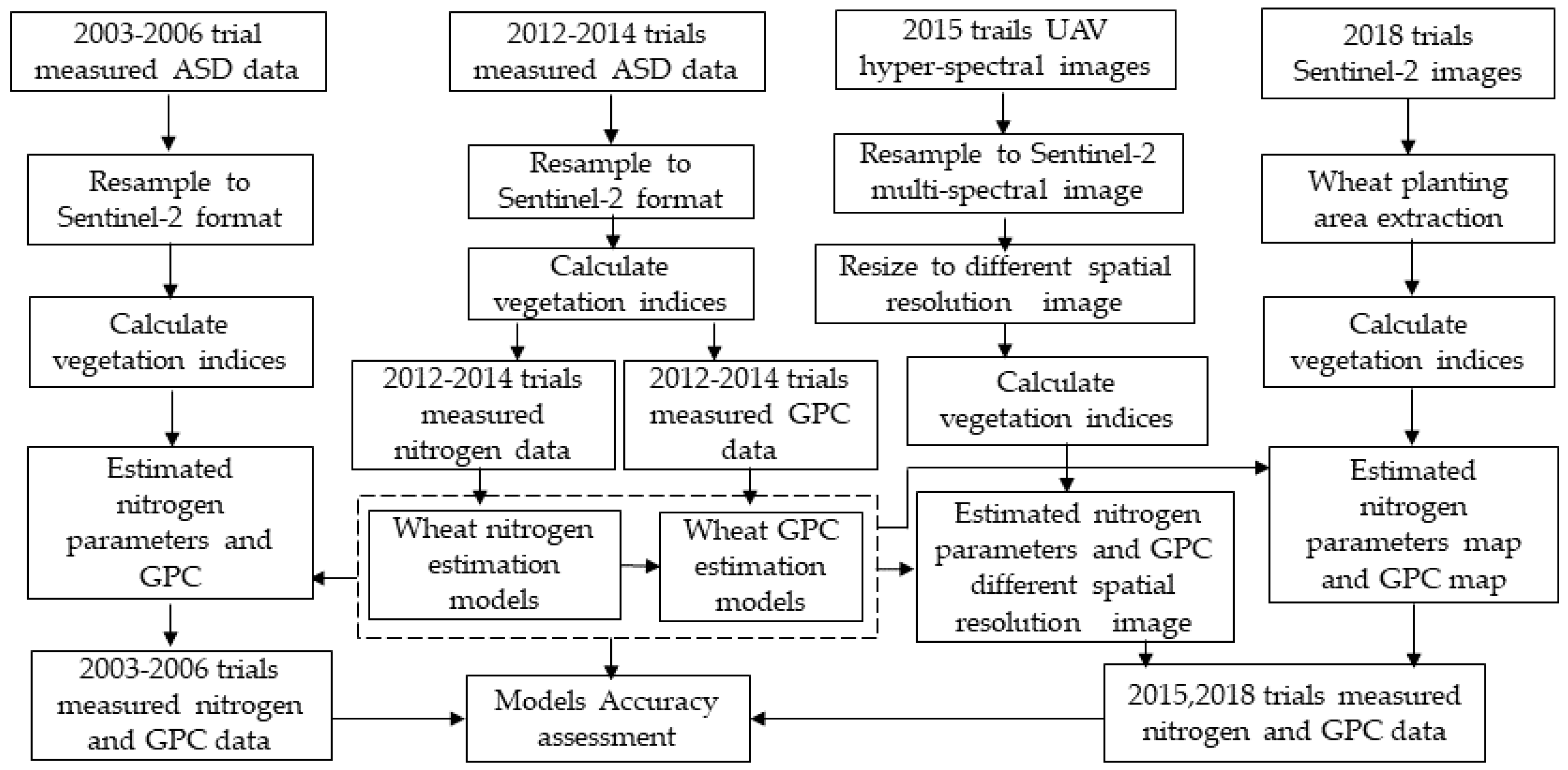
This paper aims to investigate the feasibility of using VIs derived from Sentinel-2A/2B (with an emphasis on the red-edge band) to detect the wheat N status and, furthermore, to quantitatively forecast the wheat grain protein of crops before they fully ripen. First, we simulated the Sentinel-2A/2B multispectral data from wheat canopy hyperspectral data for wheat nitrogen and GPC monitoring. Then, the relationship between different spectral parameters and wheat nitrogen index and wheat grain protein content was analyzed. The wheat nitrogen estimation models and GPC estimation model were then established based on the selected wheat nitrogen-sensitive spectral VIs. The estimation accuracy for the models was verified by simulated Sentinel-2 data obtained from UAV-UHD image in Trail 3 and ASD spectral data in the 2003–2006 experiment in Beijing. Next, the models were applied in the Renqiu area and verified by the real Sentinel-2A data and the ground measured data.
Figure 4 shows the workflow of the study.
2.3.1. Vegetation Index Selection
According to the spectral characteristics of winter wheat and the literature [
50,
51] and taking full advantage of the three red-edge bands carried by the Sentinel-2A/AB data, 14 vegetation indices were selected for estimating the wheat N status (
Table 4).
2.3.2. Multiple Linear Regression Model
The relationship between the spectral parameters and winter wheat N status in the anthesis stage was analyzed, followed by the analysis of the relationship between the grain proteins, wheat N nitrogen indicators, and spectral parameters. A multivariate linear regression (MLR) algorithm with fast modeling and no complicated calculation was applied to detect the wheat nitrogen status and the wheat GPC [
65]. The multiple linear regression models for wheat’s four nitrogen parameters (LNC, LNA, PNC, PNA) and GPC were established based on the simulated Sentinel-2 VIs from Trial 1 and Trial 2 data. A total of 80 experimental data were obtained in the 2012–2014 experiment, three-quarters of which were randomly selected (
n = 60) for modeling, and the remaining quarter of the samples (
n = 20) was used for verification.
This study mainly uses the Tensor Flow framework to achieve regression and prediction and combines the Stochastic Gradient Descent algorithm to train the model. The square loss function is used as the loss function in the training. As shown in Equation (10), where
represents the observed value,
represents the predicted value, and
n is the number of training samples:
2.3.3. Accuracy Verification
The MLR models’ estimated accuracy for the N parameter and GPC were evaluated by the simulated Sentinel-2 VIs from Trial 3 to Trial 6 and then by the real Sentinel-2B data from Trial 7. The relevant model accuracy indicators were evaluated by the normalized root mean squared error (nRMSE) [
66,
67,
68,
69]. When describing the accuracy of the verification model using nRMSE, a significant difference limit is generally given; for example, the accuracy is considered excellent when nRMSE < 10%, good if 10% ≤ nRMSE < 20%, acceptable if 20% ≤ nRMSE < 30%, and poor if nRMSE ≥ 30% [
69].
4. Discussion
Grain protein content is determined by cultivar selection, fertilization, irrigation, and environmental factors [
72,
73,
74,
75,
76,
77]. However, the main factor in determining the grain protein content may be the product of the nitrogen accumulation at the anthesis stage and the nitrogen transfer efficiency to the grain [
3]. This study revealed that the wheat nitrogen parameters at the anthesis stage and the wheat GPC under strict nitrogen control and field management show a significant relationship. The correlation coefficients between wheat PNA, PNC, LNA, LNC, and GPC were all very significant (
p < 0.01). These results coincide with the previous study by Zhenhai Li et al. [
78]. Our study also indicated that correlation coefficients between the wheat PNA, PNC, LNA, LNC, and the wheat GPC for experiments in the Beijing suburbs from Trials 3–6 were −0.145, 0.240, 0.314, and −0.142, respectively. Apparently, there are many factors that influence the nitrogen transfer efficiency from leaf to grain during the grain filling stage, such as field irrigation and environmental factors, including the moisture or heat stress across a large area.
Our study also revealed that the correlation coefficients between the spectrum VIs and wheat nitrogen parameters collected in the National Experiment Station for Precision Agriculture (Trial 1 and Trial 2;
Table 5) were more significant than those collected in the Beijing suburbs in Trials 4–7. The possible reasons for this phenomenon are that wheat canopy spectrum data collected in a farm were controlled within 1–2 h, so the spectrum difference caused by variations in the Sun elevation angle, Sun azimuth, and atmospheric conditions was limited in the experiment. In contrast, it took more than 5 h when the spectrum data were collected in the experiment fields located in Beijing suburbs; the atmospheric conditions varied very quickly when the researchers moved from one field to another field. Although the wheat canopy spectrum calibration was performed before and after the measurements using a calibration plate, the spectrum difference caused by atmospheric conditions apparently affected the precision of wheat nitrogen detection by the spectrum data.
This study reveals that there will be more challenges for detecting the cereal nitrogen status and grain quality monitoring at the regional scale through field canopy remote sensing techniques. The wheat plant nitrogen status and GPC are affected by many factors, such as soil nutrition, weather conditions, and field management. These factors, along with cultivars, contribute to the spatial variability of the crop nitrogen status and GPC. Satellite imaging may be helpful to monitor the crop growth and to predict the wheat GPC for large areas and to untangle the aforementioned factors.
Accumulation of plant nitrogen is the only direct resource for grain protein, which forms when nitrogen is physically transferred into grains at the grain filling stage. Such a relationship implies a correlation between the plant nitrogen content and the grain protein content. The results from this study demonstrate that the grain protein content was positively correlated to the leaf nitrogen content at the anthesis stage at the 99.9% significance level. The method that indirectly establishes the relationship between the spectral parameters and the grain protein content through agronomic parameters as an intermediate variable is more mechanistic, and the model has higher stability and scalability, which has become a research hotspot in recent years [
79]. As a bridge connecting the spectral parameters and the grain protein content, agronomic parameters must not only reflect the level of grain protein content but also have a significant correlation with spectral parameters. Chemura et al. [
80] have assessed the feasibility of Sentinel-2 MSI spectral bands and vegetation indices in empirical estimation of coffee foliar N content at landscape level with Sentinel-2 data; results showed that coffee foliar N is related to Sentinel-2 MSI B4, B6, B7, B8 and B12 bands, and relative vegetation indices were more related to coffee foliar N, combining optimized bands and vegetation indices produced the best results in coffee foliar N modelling (
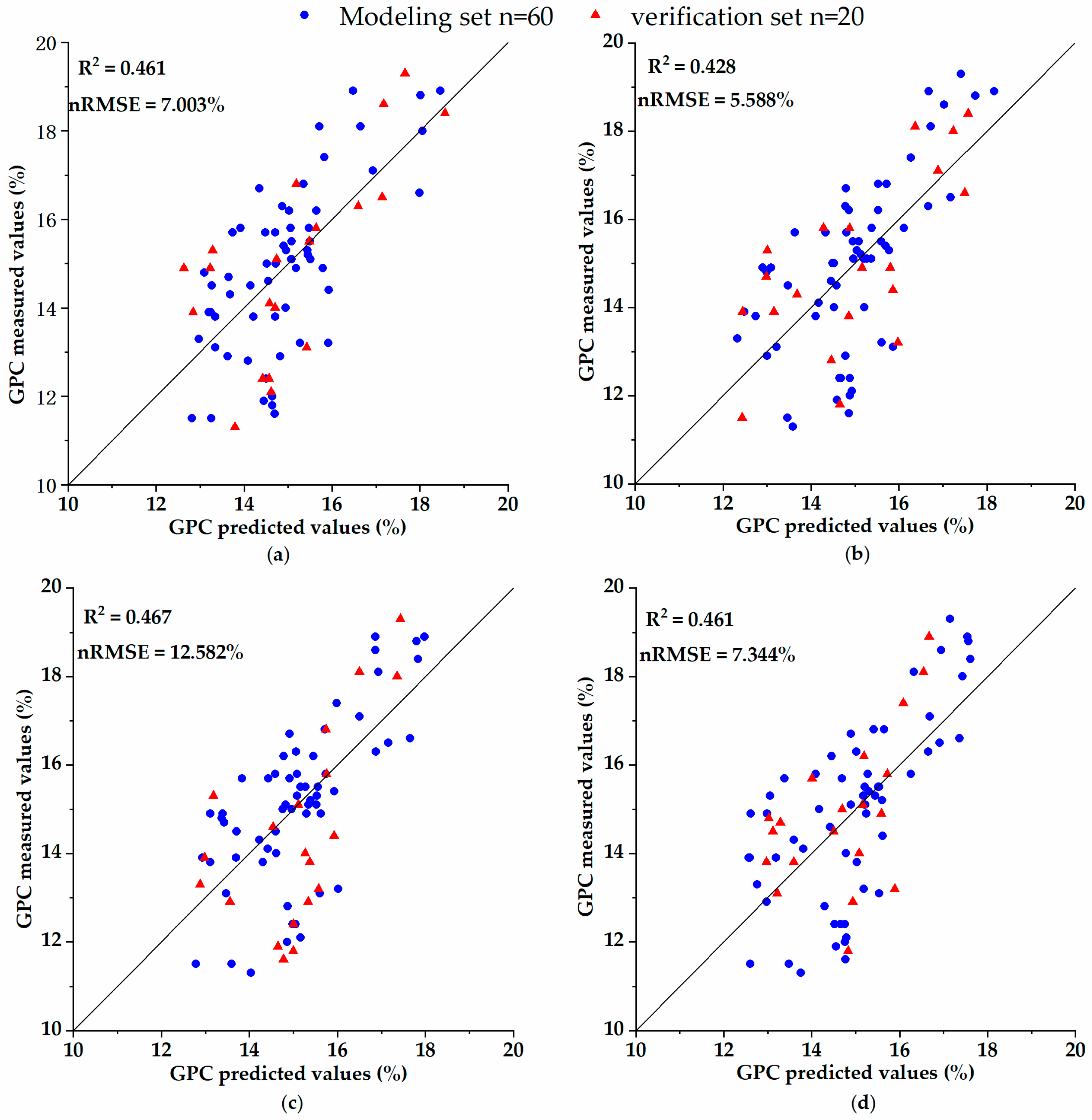
= 0.78, RMSE = 0.23). Since the quality of wheat is determined by many factors, considering the correlation between the spectral parameters and the grain content of wheat protein in previous research, in this study, the spectral parameters were added as the inversion parameters in the process of inverting the grain protein content combined with agronomic parameters in order to improve the inversion accuracy of the grain protein content. The result shows that the
of the multi-linear regression inversion models of grain protein quality were up to 0.467 and the minimum nRMSE was 7.003%, which shows that the inversion model has high precision and reliability.
Previous studies on the prediction of grain protein content in a large area using satellite data are rare and most of the related studies are based on the spectral parameters/grain protein content model. Changwei Tan et al. [
81] analyzed the quantitative relationship between satellite remote sensing variables and winter wheat grain protein content and constructed an inversion model of winter wheat grain protein content based on the multi-vegetation index using the partial least squares method and Landsat TM images. The root mean square error (RMSE) of the model was 0.37% and the coefficient of determination (
) was 0.642, and thus the inversion effect was ideal. This paper attempted to apply the inversion model of spectral parameters/agronomic parameters/grain protein content to Sentinel-2A/2B images to achieve a wide-range remoting sensing prediction of nitrogen nutrition parameters and grain protein content. The results show that the complex model with agronomic parameters still has high precision and reliability, and the mechanism and stability of the model were greatly increased.
There are still some shortcomings in this study. Because of factors such as atmospheric aerosols and different sensor types, there is a certain deviation between the reflectance value of the simulated Sentinel-2A/2B data and the actual Sentinel-2A/2B image data, which is one of the main reasons for the error in the inversion results when using the model to invert the satellite data.
Previous study indicates that for homogeneous land surface or linear algorithms, remote sensing algorithms can be scaled up or down without any error. In this study, although the winter wheat nitrogen nutrition parameters are estimated by near-linear models, some vegetation indices used in those models are calculated by nonlinear algorithm (
Table 4). There must be subpixel scale heterogeneity effects when using those models to estimate wheat nitrogen status through UAV or Sentinel 2 images. We will analyze the effects caused by the VIs and seek to utilize proper approach to correct spatial scale effects in the future studies.
In addition, in 2018, there were inconsistencies in the anthesis stage of the 20 plots in the Renqiu area. The actual farmland wheat sampling date was from 5 May to 10 May. There is a difference between the wheat nitrogen information of the relevant sample and the satellite acquisition date (8 May 2018), which also led to an incomplete correspondence between the satellite spectral information and the crop nitrogen information, which affected the final GPC inversion results. Therefore, eliminating the impacts of cultivation, climate, and other factors in different plots in the large area is also a problem that needs to be solved in further studies for the development of wheat nitrogen and GPC remote sensing at a regional scale.