Leaf-Level Spectral Fluorescence Measurements: Comparing Methodologies for Broadleaves and Needles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Protocol
2.2. Description of Methods
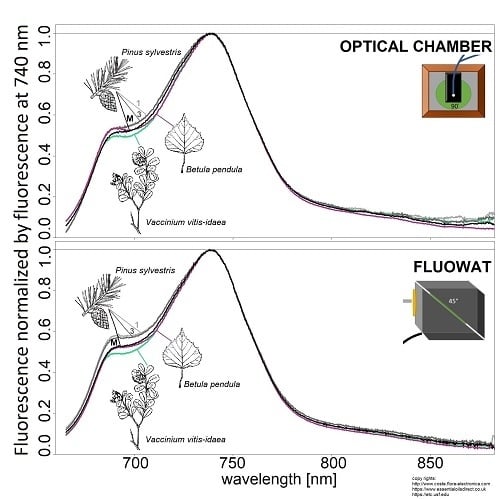
2.2.1. Optical Chamber
2.2.2. FluoWat
2.2.3. Integrating Sphere
2.3. Baseline Correction
2.4. Data Analysis and Presentation
3. Results
3.1. Baseline Correction
3.2. Comparison of Measurement Protocols
3.3. Comparison of Needle Arrangements
3.4. Comparison of Leaf Morphology
4. Discussion
4.1. Baseline Correction and the Red/Far-Red Ratio
4.2. Measurement Protocols
4.3. Needle Arrangements and Morphology Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davidson, M.; Berger, M.; Moya, I.; Moreno, J.; Laurila, T.; Stoll, M.-P.; Miller, J. Mapping Photosynthesis from Space—A New Vegetation-Fluorescence Technique. Eur. Space Agency Bull. 2003, 116, 34–37. [Google Scholar]
- Moya, I.; Camenen, L.; Evain, S.; Goulas, Y.; Cerovic, Z.G.; Latouche, G.; Flexas, J.; Ounis, A. A new instrument for passive remote sensing: 1. Measurements of sunlight-induced chlorophyll fluorescence. Remote Sens. Environ. 2004, 91, 186–197. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berni, J.A.; Suárez, L.; Sepulcre-Cantó, G.; Morales, F.; Miller, J. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- Guanter, L.; Frankenberg, C.; Dudhia, A.; Lewis, P.E.; Gómez-Dans, J.; Kuze, A.; Suto, H.; Grainger, R.G. Retrieval and global assessment of terrestrial chlorophyll fluorescence from GOSAT space measurements. Remote Sens. Environ. 2012, 121, 236–251. [Google Scholar] [CrossRef]
- Frankenberg, C.; Fisher, J.B.; Worden, J.; Badgley, G.; Saatchi, S.S.; Lee, J.E.; Toon, G.C.; Butz, A.; Jung, M.; Kuze, A.; Yokota, T. New global observations of the terrestrial carbon cycle from GOSAT: Patterns of plant fluorescence with gross primary productivity. Geophys. Res. Lett. 2011, 38, L17706. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.-E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Frankenberg, C.; Wood, J.D.; Schimel, D.S.; Jung, M.; Guanter, L.; Drewry, D.T.; Verma, M.; Porcar-Castell, A.; Griffis, T.J.; Gu, L.; Magney, T.S.; Köhler, P.; Evans, B.; Yuen, K. OCO-2 advances photosynthesis observation from space via solar-induced chlorophyll fluorescence. Science 2017, 358. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Rossin, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Rascher, U.; Agati, G.; Alonso, L.; Cecchi, G.; Champagne, S.; Colombo, R.; Damm, A.; Daumard, F.; Miguel, E. CEFLES2: The remote sensing component to quantify photosynthetic efficiency from the leaf to the region by measuring sun-induced fluorescence in the oxygen absorption bands. Biogeosciences 2009, 6, 1181–1198. [Google Scholar] [CrossRef]
- Malenovský, Z.; Mishra, K.B.; Zemek, F.; Rascher, U.; Nedbal, L. Scientific and technical challenges in remote sensing of plant canopy reflectance and fluorescence. J. Exp. Bot. 2009, 60, 2987–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J. Review Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Remote sensing of chlorophyll fluorescence in oceanography and terrestrial vegetation: An introduction. In Applications of Chlorophyll Fluorescence in Photosynthesis Research, Stress Physiology, Hydrobiology and Remote Sensing; Springer: Dordrecht, The Netherlands, 1988; pp. 287–297. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and hyperspectral airborne experiments. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Govindjee. Chlorophyll a Fluorescence—A Signature of Photosynthesis; Springer: Berlin, Germany, 2004. [Google Scholar]
- Agati, G.; Cerovic, Z.G.; Moya, I. The effect of decreasing temperature up to chilling values on the in vivo F685/F735 chlorophyll fluorescence ratio in Phaseolus vulgaris and Pisum savitum: The role of photosystem I contribution to the 735 nm fluorescence band. Photochem. Photobiol. 2000, 72, 75–84. [Google Scholar] [CrossRef]
- Valentini, R.; Cecchi, G.; Mazzinghi, P.; Scarascia Mugnozza, G.; Agati, G.; Bazzani, M.; De Angelis, P.; Fusi, F.; Matteucci, G.; Raimondi, V. Remote Sensing of Chlorophyll a Fluorescence of Vegetation Canopies: 2. Physiological Significance of Fluorescence Signal In Response to Environmental Stresses. Remote Sens. Environ. 1994, 47, 29–35. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Hermans, I.; Valcke, R.; Veroustraete, F.; Moreno, J.; Samson, R. A field study on solar-induced chlorophyll fluorescence and pigment parameters along a vertical canopy gradient of four tree species in an urban environment. Sci. Total Environ. 2014, 466–467, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Butler, W.L. Fluorescence emission spectra of photosystem I, photosystem II and the light-harvesting chlorophyll a/b complex of higher plants. Biochim. Biophys. Acta 1977, 462, 307–313. [Google Scholar] [CrossRef]
- Pfündel, E. Estimating the contribution of Photosystem I to total leaf chlorophyll fluorescence. Photosynth. Res. 1998, 56, 185–195. [Google Scholar] [CrossRef]
- Franck, F.; Janeau, P.; Popovic, R. Resolution of the Photosystem I and Photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochim. Biophys. Acta 2002, 1556, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Lavorel, J. Hétérogénéité de la chlorophylle in vivo I. Spectres d’émission de fluorescence (Heterogeneity of chlorophyll in vivo I. Fluorescence spectra). Biochem. Biophys. Acta 1962, 60, 510–523. [Google Scholar] [CrossRef]
- Weis, E. Light- and temperature-induced changes in the distribution of excitation energy between Photosystem I and Photosystem II in spinach leaves. Biochim. Biophys. Acta (BBA) Bioenerg. 1985, 807, 118–126. [Google Scholar] [CrossRef]
- Demming, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in levels of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Govindjee. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar]
- FAO. Available online: http://www.fao.org/docrep/013/i1757e/i1757e.pdf (accessed on 21 May 2018).
- Daughtry, C.S.T.; Biehl, L.L.; Ranson, K.J. A new technique to measure the spectral properties of conifer needles. Remote Sens. Environ. 1989, 27, 81–91. [Google Scholar] [CrossRef]
- Harron, J.W.; Miller, J.R. An alternate methodology for reflectance and transmittance measurements of conifer needles. Proc. Can. Remote Sens. Symp. 1995, 2, 654–661. [Google Scholar]
- Yáñez-Rausell, L.; Schaepman, M.E.; Clevers, J.G.P.W.; Malenovsky, Z. Minimizing measurement uncertainties of coniferous needle-leaf optical properties. Part I: Methodological review. IEEE J. Sel. Top. Appl. Earth Observ. Remote Sens. 2013, 99, 1–7. [Google Scholar] [CrossRef]
- Middleton, E.M.; Chan, S.S.; Rusin, R.J.; Mitchell, S.K. Optical Properties of Black Spruce and Jack Pine Needles at BOREAS Sites in Saskatchewan, Canada. CJRS 1997, 23, 108–119. [Google Scholar] [CrossRef]
- Williams, D.L. A comparison of spectral reflectance properties at the needle, branch, and canopy level for selected Conifer species. Remote Sens. Environ. 1991, 35, 79–93. [Google Scholar] [CrossRef]
- Middleton, E.M.; Chan, S.S.; Mesarch, M.A.; Walter-Shea, E.A. Revised measurement methodology for spectral optical properties of conifer needles. Proc. IEEE IGARSS 1996, 1005–1009. [Google Scholar] [CrossRef]
- Olascoaga, B.; Mac Arthur, A.; Atherton, J.; Porcar-Castell, A. A comparison of methods to estimate photosynthetic light absorption in leaves with contrasting morphology. Tree Physiol. 2016, 36, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesarch, M.A.; Walter-Shea, E.A.; Asner, G.P.; Middleton, E.M.; Chan, S.S. A revised measurement methodology for conifer needles spectral optical properties: Evaluating the influence of gaps between elements. Remote Sens. Environ. 1999, 68, 177–192. [Google Scholar] [CrossRef]
- Yáñez-Rausell, L.; Malenovsky, Z.; Clevers, J.G.P.W.; Schaepman, M.E. Minimizing Measurement Uncertainties of Coniferous Needle-Leaf Optical Properties, Part II: Experimental set-up and error analysis. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Alonso, L.; Gómez-Chova, L.; Vila-Francés, J.; Amorós-López, J.; Guanter, L.; Calpe, J.; Moreno, J. Sensitivity analysis of the Fraunhofer Line Discrimination method for the measurement of chlorophyll fluorescence using a field spectroradiometer. IEEE Int. Geosci. Rem. Sens. Symp. 2007, 1–12, 3756–3759. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Atherton, J.; Olascoaga, B.; Alonso, L.; Porcar-Castell, A. Spatial Variation of Leaf Optical Properties in a Boreal Forest Is Influenced by Species and Light Environment. Front. Plant Sci. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Solanki, T.; Aphalo, P.J.; Neimane, S.; Hartikainen, S.M.; Pieristè, M.; Porcar-Castell, A.; Atherton, J.; Heikkilä, A.; Robson, M.T. UV-screening and springtime recovery of photosynthetic capacity in leaves of Vaccinium vitis-idaea above and below the snow pack. Plant Physiol. Biochem. 2019, 134, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Hermans, I.; Delegido, J.; Veroustraete, F.; Valcke, R.; Moreno, J.; Samson, R. Upward and downward solar-induced chlorophyll fluorescence yield indices of four tree species as indicators of traffic pollution in Valencia. Environ. Pollut. 2013, 173, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hák, R.; Lichtenthaler, H.K.; Rinderle, U. Decrease of the chlorophyll fluorescence ratio F690/F730 during greening and development of leaves. Radiat. Environ. Biophys. 1990, 29, 329–336. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Hák, R.; Rinderle, U. The chlorophyll fluorescence ratio F690/F730 in leaves of different chlorophyll content. Photosynth. Res. 1990, 25, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Mazzinghi, P.; Fusi, F.; Ambrosini, I. The F685/F730 Chlorophyll fluorescence ratio as a tool in plant physiologu: Response to physiological and environmental factors. J. Plant Physiol. 1995, 145, 228–238. [Google Scholar] [CrossRef]
- Agati, G.; Mazzinghi, P.; Lippucci di Paola, M.; Fusi, F.; Cecchi, G. The F685/F730 chlorophyll fluorescence ratio as indicator of chilling stress in plants. J. Plant Physiol. 1996, 148, 384–390. [Google Scholar] [CrossRef]
- Genty, B.; Wonders, J.; Baker, N.R. Non-photochemical quenching of F0 in leaves is emission wavelength dependent: Consequences for quenching analysis and its interpretation. Photosynth. Res. 1990, 26, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Palombi, L.; Cecchi, G.; Lognoli, D.; Raimondi, V.; Toci, G.; Agati, G. A retrieval algorithm to evaluate the Photosystem I and Photosystem II spectral contributions to leaf chlorophyll fluorescence at physiological temperatures. Photosynth. Res. 2011, 108, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Magney, T.S.; Frankenberg, C.; Fisher, J.B.; Sun, Y.; North, G.B.; Davis, T.S.; Kornfeld, A.; Siebke, K. Connecting active to passive fluorescence with photosynthesis: A method for evaluating remote sensing measurements of Chl fluorescence. New Phytol. 2017, 25, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Lukeš, P.; Stenberg, P.; Rautiainen, M.; Mõttus, M.; Vanhatalo, K.M. Optical properties of leaves and needles for boreal tree species in Europe. Remote Sens. Lett. 2013, 4, 667–676. [Google Scholar] [CrossRef]







| Feature | Optical Chamber | FluoWat | Integrating Sphere |
|---|---|---|---|
| Light source | Ocean Optics® HL-2000 (halogen) | ASD® RTS-3ZC dedicated light source (halogen) | |
| Light intensity at leaf surface | 50 µmol | 38 µmol | |
| Spectrometer | Ocean Optics® USB2000+ (range: 200–1100 nm, FWHM (600–800 nm): 1.5–1.8 nm) | ||
| Optical fiber | Ocean Optics® R600-7-VIS-125F (600 µm) | ASD (600 µm) | |
| Background | black tape | FW light trap | IS light trap |
| Filter | Thorlabs® (cut-off wavelength 650 nm, optical density = 4) | Edmund Optics® (cut-off wavelength 650 nm, optical density = 4) | |
| Integration Time | Optical Chamber | FluoWat | Integrating Sphere | |
|---|---|---|---|---|
| short | Integration time (ms) | 14 | 100 | 1000 |
| Number of averaged spectra | 25 | 10 | 10 | |
| long | Integration time (ms) | 300 | 15,000 | 60,000 |
| Number of averaged spectra | 1 | 1 | 1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajewicz, P.A.; Atherton, J.; Alonso, L.; Porcar-Castell, A. Leaf-Level Spectral Fluorescence Measurements: Comparing Methodologies for Broadleaves and Needles. Remote Sens. 2019, 11, 532. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050532
Rajewicz PA, Atherton J, Alonso L, Porcar-Castell A. Leaf-Level Spectral Fluorescence Measurements: Comparing Methodologies for Broadleaves and Needles. Remote Sensing. 2019; 11(5):532. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050532
Chicago/Turabian StyleRajewicz, Paulina A., Jon Atherton, Luis Alonso, and Albert Porcar-Castell. 2019. "Leaf-Level Spectral Fluorescence Measurements: Comparing Methodologies for Broadleaves and Needles" Remote Sensing 11, no. 5: 532. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050532