A Pilot Study to Estimate Forage Mass from Unmanned Aerial Vehicles in a Semi-Arid Rangeland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Image Acquisition
2.3. Field Data Collection
2.4. Image Processing and Volume Estimation
2.5. Statistical Analyses
2.6. Forage Mass Estimation
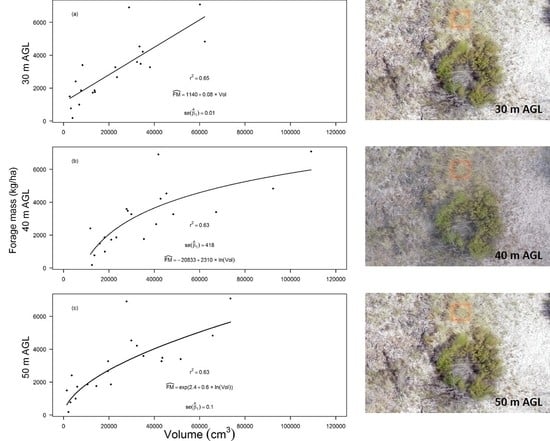
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, V.G.; Batello, C.; Berretta, E.J.; Hodgson, J.; Kothmann, M.; Li, X.; McIvor, J.; Milne, J.; Morris, C.; Peeters, A.; et al. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 2011, 66, 2–28. [Google Scholar] [CrossRef]
- Brummer, J.E.; Nichols, J.T.; Engel, R.K.; Eskridge, K.M. Efficiency of different quadrat sizes and shapes for sampling standing crop. J. Range Manag. 1994, 47, 84–89. [Google Scholar] [CrossRef]
- Byrne, K.; Lauenroth, W.; Adler, P.; Byrne, C. Estimating Aboveground Net Primary Production in Grasslands: A Comparison of Nondestructive Methods. Rangel. Ecol. Manag. 2011, 64, 498–505. [Google Scholar] [CrossRef]
- Catchpole, W.R.; Catchpole, E.A. Stratified Double Sampling of Patchy Vegetation to Estimate Biomass. Biometrics 1993, 49, 295–303. [Google Scholar] [CrossRef]
- Zhang, H.; Sum, Y.; Chang, L.; Qin, Y.; Du, J.; Yi, S.; Wang, Y. Estimation of grassland Canopy Height and Aboveground Biomass at the Quadrat Scale Using Unmanned Aerial Vehicle. Remote Sens. 2018, 10, 851. [Google Scholar] [CrossRef] [Green Version]
- Gillan, J.K.; McClaran, M.P.; Swetnam, T.L.; Heilman, P. Estimating forage utilization with drone-based photogrammetric point clouds. Rangel. Ecol. Manag. 2019, 72, 575–585. [Google Scholar] [CrossRef]
- Glasscock, S.N.; Grant, W.E.; Draw, D.L. Simulation of vegetation dynamics and management strategies on south Texas, semi-arid rangeland. J. Environ. Manag. 2005, 75, 379–397. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Briske, D.D.; Smeins, F.E. Herbaceous vegetation change in variable rangeland environments: The relative contribution of grazing and climatic variability. Appl. Veg. Sci. 2001, 4, 177–188. [Google Scholar] [CrossRef]
- Chen, W.; Blain, D.; Li, J.; Koehler, K.; Fraser, R.; Zhang, Y.; Leblanc, S.; Olthof, I.; Wang, J.; McGovern, M. Biomass measurements and relationships with Landsat-7/ETM+ and JERS-1/SAR data over Canada’s western sub-arctic and low arctic. Int. J. Remote Sens. 2009, 30, 2355–2376. [Google Scholar] [CrossRef]
- Gibb, M.J.; Ridout, M.S. The fitting of frequency distributions to height measurements on grazed swards. Grass Forage Sci. 1986, 41, 247–249. [Google Scholar] [CrossRef]
- Holechek, J.L.; Pieper, R.D.; Herbel, C.H. Range Management: Principles and Practices, 6th ed.; Pearson: New York, NY, USA, 2011; pp. 169–201. [Google Scholar]
- Illius, A.W.; Wood-Gush, D.G.M.; Eddison, J.C. A study of the foraging behavior of cattle grazing patchy swards. Biol. Behav. 1987, 12, 33–44. [Google Scholar]
- Tsutsumi, M.; Itano, S.; Shiyomi, M. Number of Samples Required for Estimating Herbaceous Biomass. Rangel. Ecol. Manag. 2007, 60, 447–452. [Google Scholar] [CrossRef]
- Bonham, C.D. Measurements for Terrestrial Vegetation, 2nd ed.; John Wiley and Sons: West Sussex, UK, 2013; pp. 175–199. [Google Scholar]
- Laliberte, A.S.; Herrick, J.E.; Rango, A.; Winters, C. Acquisition, orthorectification, and object-based classification of unmanned aerial vehicle (UAV) imagery for rangeland monitoring. Photogramm. Eng. Remote Sens. 2010, 76, 661–672. [Google Scholar] [CrossRef]
- Castillo-Santiago, M.; Ricker, M.; Bernardus, J. Estimation of tropical forest structure from SPOT5 satellite images. Int. J. Remote Sens. 2010, 31, 2767–2782. [Google Scholar] [CrossRef]
- Everitt, J.H.; Anderson, G.L.; Escobar, D.E.; Davis, M.R.; Spencer, N.R.; Andrascik, R.J. Use of remote sensing for detecting and mapping leafy spurge (Euphorbia esula). Weed Technol. 1995, 9, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.; Perotto-Baldivieso, H.L.; Hernández, F.; Grahmann, E.D.; Rideout-Hanzak, S.; Edwards, J.T.; Page, M.T.; Shedd, T.M. Quantifying the spatial and temporal distribution of tanglehead (Heteropogon contortus) on South Texas rangelands. Ecol. Process. 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Sinha, P.; Taylor, S.; Alqurashi, A. Review of the use of remote sensing for biomass estimation to support renewable energy generation. J. Appl. Remote Sens. 2015, 9, 097696. [Google Scholar] [CrossRef]
- Dube, T.; Mutanga, O. Evaluating the utility of the medium-spatial resolution Landsat 8 multispectral sensor in quantifying aboveground biomass in uMgeni catchment, South Africa. ISPRS J. Photogramm. Remote Sens. 2015, 101, 36–46. [Google Scholar] [CrossRef]
- Grant, K.M.; Johnson, D.L.; Hildebrand, D.V.; Peddle, D.R. Quantifying biomass production on rangeland in southern Alberta using SPOT imagery. Can. J. Remote Sens. 2013, 38, 695–708. [Google Scholar] [CrossRef]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Shoko, C.; Mutanga, O.; Dube, T. Progress in the remote sensing of C3 and C4 grass species aboveground biomass over time and space. ISPRS J. Photogramm. Remote Sens. 2016, 120, 13–24. [Google Scholar] [CrossRef]
- Manfreda, S.; McCabe, M.F.; Miller, P.E.; Lucas, R.; Madrigal, V.P.; Mallinis, G.; Ben Dor, E.; Helman, D.; Estes, L.; Ciraolo, G.; et al. On the Use of Unmanned Aerial Systems for Environmental Monitoring. Remote Sens. 2018, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Cunliffe, A.M.; Brazier, R.E.; Anderson, K. Ultra-fine grain landscape-scale quantification of dryland vegetation structure with drone-acquired structure-from-motion photogrammetry. Remote Sens. Environ. 2016, 183, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Gillan, J.K.; Karl, J.W.; van Leeuwen, W.J.D. Integrating drone imagery with existing rangeland monitoring programs. Environ. Monit. Assess. 2020, 192, 269. [Google Scholar] [CrossRef] [PubMed]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (CSMS) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef] [Green Version]
- Tilly, A.N.; Hoffmeister, D.; Cao, Q.; Huang, S.; Lenz-Wiedemann, V.; Miao, Y.; Bareth, G. Multitemporal crop surface models: Accurate plant height measurement and biomass estimation with terrestrial laser scanning in paddy rice. J. Appl. Remote Sens. 2014, 8, 083671. [Google Scholar] [CrossRef]
- Li, W.; Niu, Z.; Chen, H.; Li, D.; Wu, M.; Zhao, W. Remote estimation of canopy height and aboveground biomass of maize using high-resolution stereo images from a low-cost unmanned aerial vehicle system. Ecol. Indic. 2016, 67, 637–648. [Google Scholar] [CrossRef]
- Souza, C.H.W.D.; Lamparelli, R.A.C.; Rocha, J.V. Height estimation of sugarcane using an unmanned aerial system UAS based on structure from motion SFM point clouds. Int. J. Remote Sens. 2017, 38, 2218–2230. [Google Scholar] [CrossRef]
- Wallace, L.; Lucieer, A.; Malenovský, Z.; Turner, D.; Vopěnka, P. Assessment of forest structure using two UAV techniques: A comparison of airborne laser scanning and structure from motion (SfM) point clouds. Forests 2016, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Ku, N.-W.; Popescu, S.; Ansley, R.J.; Perotto-Baldivieso, H.L. Assessment of available rangeland woody plant biomass with a terrestrial lidar system. Photogramm. Eng. Remote Sens. 2012, 78, 349–361. [Google Scholar] [CrossRef]
- Ten Harkel, J.; Bartholomeus, H.; Kooistra, L. Biomass and Crop Height Estimation of Different Crops Using UAV-Based Lidar. Remote Sens. 2019, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, A.; Snelgrove, T.; Riojas, G.; Schofield, L.; Campbell, T.A. Cattle ranching in the “Wild Horse Desert”—Stocking rate, rainfall, and forage responses. Rangelands 2020, 42, 31–42. [Google Scholar] [CrossRef]
- Despain, D.W.; Smith, E.L. The comparative yield method for estimating range production. In Some Methods for Monitoring Rangelands and Other Natural Area Vegetation; Rule, G.B., Ed.; Arizona Cooperative Extension Publication 190043: Tucson, AZ, USA, 1987; pp. 49–90. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Texas Parks and Wildlife. Ecoregions of Texas. 2018. Available online: https://tpwd.texas.gov/education/hunter-education/online-course/wildlife-conservation/texas-ecoregions (accessed on 6 October 2018).
- U.S. Climate Data. Climate Freer-Texas. 2018. Available online: https://www.usclimatedata.com/climate/freer/texas/united-states/ustx0589 (accessed on 4 October 2018).
- Web Soil Survey. AOI of Study Site. Available online: https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 19 June 2020).
- Ecological Site Description Catalog. Available online: https://edit.jornada.nmsu.edu/page?content=class&catalog=3&spatial=163&class=8418 (accessed on 19 June 2020).
- Hatch, S.L.; Umphres, K.C.; Ardon, A.J. Field Guide to Common Texas Grasses; Texas A&M University Press: College Station, TX, USA, 2015. [Google Scholar]
- USDA; NRCS. The PLANTS Database. 2019. Available online: http://plants.usda.gov (accessed on 5 November 2018).
- DJI. Phantom 4 Pro Information. 2018. Available online: https://www.dji.com/phantom-4-pro/info. (accessed on 20 October 2018.).
- Garza, B.N.; Ancona, V.; Enciso, J.; Perotto-Baldivieso, H.L.; Kunta, M.; Simpson, C. Quantifying Citrus Tree Health Using True Color UAV Images. Remote Sens. 2020, 12, 170. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Ablanedo, E.; Chandler, J.H.; Rodríguez-Pérez, J.R.; Ordóñez, C. Accuracy of Unmanned Aerial Vehicle (UAV) and SfM Photogrammetry Survey as a Function of the Number and Location of Ground Control Points Used. Remote Sens. 2018, 10, 1606. [Google Scholar] [CrossRef] [Green Version]
- Pieper, R.D. Rangeland vegetation productivity and biomass. In Vegetation Science Applications for Rangeland Analysis and Management; Tueller, P.T., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 449–467. [Google Scholar]
- Pix4D. Pix4Dmapper. 2016. Available online: https://www.pix4d.com/product/pix4dmapper-photogrammetry-software (accessed on 10 February 2019).
- Cubero-Castan, M.; Schneider-Zapp, K.; Bellomo, M.; Shi, D.; Rehak, M.; Strecha, C. Assessment of the Radiometric Accuracy in a Target Less Work Flow Using Pix4D Software. In Proceedings of the 2018 9th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Amsterdam, The Netherlands, 23 September 2018; pp. 1–4. [Google Scholar]
- Burns, J.H.R.; Delparte, D. Comparison of commercial structure-from-motion photogrammetry software used for underwater three-dimensional modeling of coral reef environments. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2017, XLII-2/W3, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, X.; Ai, G.; Zhang, Y.; Zuo, Y. Generating a High-Precision True Digital Orthophoto Map based on UAV images. ISPRS Int. J. Geo-Inf. 2018, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Gujarati, D.N.; Porter, D.C. Basic Econometrics, 5th ed.; McGraw Hill Inc.: New York, NY, USA, 2009. [Google Scholar]
- Sokal, R.R.; James, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- White, H.A. Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Walter, J.; Edwards, J.; McDonald, G.; Kuchel, H. Photogrammetry for the estimation of wheat biomass and harvest index. Field Crop. Res. 2018, 216, 165–174. [Google Scholar] [CrossRef]
- Hanselka, C.W.; White, L.D.; Holechek, J.L. Using forage harvest efficiency to determine stocking rate. Tex. Coop. Ext. 2014, E-128, 2. [Google Scholar]
- Ortega-S., J.A.; Bryant, F.C. Cattle Management to Enhance Wildlife Habitat; Management Bulletin No. 6; Caesar Kleberg Wildlife Research Institute: Kingsville, TX, USA, 2005; 12p. [Google Scholar]
- Juecker, T.; Caspersen, J.; Chave, J.; Antin, C.; Barbier, N.; Bongers, F.; Dalponte, M.; Ewijk, K.Y.; Forrester, D.I.; Haeni, M.; et al. Allometric equations for integrating remote sensing imagery into forest monitoring programmes. Glob. Chang. Biol. 2017, 23, 177–190. [Google Scholar] [CrossRef] [PubMed]




| Species Canopy Cover (%) | ||||||
|---|---|---|---|---|---|---|
| Quadrat | Pink Pappusgrass | Common Broomweed | Tumble Lovegrass | Multiflowered False Rhodesgrass | Plains Bristlegrass | Sand Mat |
| 1 | 65 | 0 | 0 | 0 | 0 | 0 |
| 2 | 20 | 0 | 0 | 0 | 0 | 0 |
| 3 | 70 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 40 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 70 | 0 | 0 |
| 6 | 30 | 0 | 0 | 0 | 0 | 0 |
| 7 | 40 | 0 | 0 | 0 | 0 | 0 |
| 8 | 15 | 25 | 0 | 0 | 0 | 0 |
| 9 | 80 | 0 | 0 | 0 | 0 | 0 |
| 10 | 100 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 45 | 0 | 0 | 0 |
| 12 | 15 | 65 | 0 | 0 | 0 | 0 |
| 13 | 25 | 0 | 0 | 0 | 0 | 0 |
| 14 | 95 | 0 | 0 | 0 | 0 | 0 |
| 15 | 75 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 30 | 0 |
| 17 | 65 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 100 | 0 | 15 |
| 19 | 40 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 15 | 0 | 0 | 0 |
| Flight Altitude | Road Area | Treated Area | ||
|---|---|---|---|---|
| Average Volume (cm3) | Forage Mass (kg/ha) | Average Volume (cm3) | Forage Mass (kg/ha) | |
| 30 m AGL | 3423 | 1413 | 6599 | 1667 |
| 40 m AGL | 3446 | 7688 | ||
| 50 m AGL | 3520 | 1479 | 5293 | 1890 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiMaggio, A.M.; Perotto-Baldivieso, H.L.; Ortega-S., J.A.; Walther, C.; Labrador-Rodriguez, K.N.; Page, M.T.; Martinez, J.d.l.L.; Rideout-Hanzak, S.; Hedquist, B.C.; Wester, D.B. A Pilot Study to Estimate Forage Mass from Unmanned Aerial Vehicles in a Semi-Arid Rangeland. Remote Sens. 2020, 12, 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12152431
DiMaggio AM, Perotto-Baldivieso HL, Ortega-S. JA, Walther C, Labrador-Rodriguez KN, Page MT, Martinez JdlL, Rideout-Hanzak S, Hedquist BC, Wester DB. A Pilot Study to Estimate Forage Mass from Unmanned Aerial Vehicles in a Semi-Arid Rangeland. Remote Sensing. 2020; 12(15):2431. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12152431
Chicago/Turabian StyleDiMaggio, Alexandria M., Humberto L. Perotto-Baldivieso, J. Alfonso Ortega-S., Chase Walther, Karelys N. Labrador-Rodriguez, Michael T. Page, Jose de la Luz Martinez, Sandra Rideout-Hanzak, Brent C. Hedquist, and David B. Wester. 2020. "A Pilot Study to Estimate Forage Mass from Unmanned Aerial Vehicles in a Semi-Arid Rangeland" Remote Sensing 12, no. 15: 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12152431