Spatiotemporal Dynamics and Environmental Controlling Factors of the Lake Tana Water Hyacinth in Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Lake Tana Hydrology
2.3. Methods
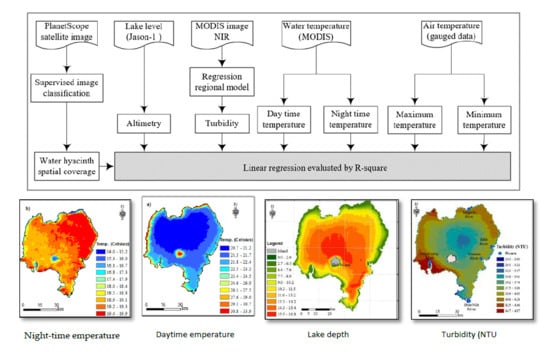
2.3.1. Spatiotemporal Water Hyacinth Distribution Mapping
2.3.2. Major Factors Affecting Water Hyacinth Spatial Coverage Dynamics
3. Results
3.1. Water Hyacinth Spatiotemporal Dynamics
3.2. Environmental Factors Plausibly Contributing to the Water Hyacinth Dynamics
3.2.1. Lake Tana Bathymetric Survey
3.2.2. Air and Water Temperature
3.2.3. Turbidity
3.2.4. Lake Level Variation
3.3. The Interplay between Environmental Factors and Water Hyacinth Coverage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penfound, W.T.; Earle, T.T. The Biology of the Water Hyacinth. Ecol. Monogr. 1948, 18, 447–472. [Google Scholar] [CrossRef]
- Reddy, K. Water hyacinth (Eichhornia crassipes) biomass production in Florida. Biomass 1984, 6, 167–181. [Google Scholar] [CrossRef]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Verma, V.; Sharma, P.K. Mapping, monitoring and conservation of Harike wetland ecosystem, Punjab, India, through remote sensing. Int. J. Remote Sens. 2001, 22, 89–98. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, X.; Ma, X.; Chen, J. Sustainable removal of formaldehyde using controllable water hyacinth. J. Clean. Prod. 2018, 181, 1–7. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Brendonck, L.; Maes, J.; Rommens, W.; Dekeza, N.; Nhiwatiwa, T.; Barson, M.; Callebaut, V.; Phiri, C.; Moreau, K.; Gratwicke, B.; et al. The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). II. Species diversity. Arch. Hydrobiol. 2003, 158, 389–405. [Google Scholar] [CrossRef]
- Cilliers, C. Biological control of water hyacinth, Eichhornia crassipes (Pontederiaceae), in South Africa. Agric. Ecosyst. Environ. 1991, 37, 207–217. [Google Scholar] [CrossRef]
- Fessehaie, R. Water hyacinth (Eichhornia crassipes): A Review of its weed status in Ethiopia. Arem (Ethiopia) 2005, 6, 105–106. [Google Scholar]
- Byrne, M.; Hill, M.; Robertson, M.; King, A.; Jadhav, A.; Katembo, N.; Wilson, J.; Brudvig, R.; Fisher, J. Integrated Management of Water Hyacinth in South Africa: Development of an integrated management plan for water hyacinth control, combining biological control, herbicidal control and nutrient control, tailored to the climatic regions of South Africa. WRC Rep. 2010, 454, 302. [Google Scholar]
- Mesfin, M.; Tudorancea, C.; Baxter, R.M. Some limnological observations on two Ethiopian hydroelectric reservoirs: Koka (Shewa administrative district) and Finchaa (Welega administrative district). Hydrobiologia 1988, 157, 47–55. [Google Scholar] [CrossRef]
- Tewabe, D. Preliminary Survey of Water Hyacinth in Lake. Glob. J. Allergy 2015, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Anteneh, W.; Tewabe, D.; Assefa, A.; Zeleke, A.; Tenaw, B.; Wassie, Y. Water Hyacinth Coverage Survey Report on Lake Tana Biosphere Reserve; Technical Report Series 2. Available online: https://welkait.com/wp-content/uploads/2017/06/Water-hacinth_Lake-Tana_Report-Series-2.pdf (accessed on 20 August 2020).
- Gebremedhin, S.; Getahun, A.; Anteneh, W.; Bruneel, S.; Goethals, P. A Drivers-Pressure-State-Impact-Responses Framework to Support the Sustainability of Fish and Fisheries in Lake Tana, Ethiopia. Sustainability 2018, 10, 2957. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.; Richardson, D.M.; Rouget, M.; Procheş, Ş.; Amis, M.A.; Henderson, L.; Thuiller, W. Residence time and potential range: Crucial considerations in modelling plant invasions. Divers. Distrib. 2007, 13, 11–22. [Google Scholar] [CrossRef]
- Priya, P.; Nikhitha, S.; Anand, C.; Nath, R.D.; Bhaskaran, K. Biomethanation of water hyacinth biomass. Bioresour. Technol. 2018, 255, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, G. Monitoring the Effects of Biological Control of Water Hyacinths Using Remotely Sensed Data: A Case Study of Bangalore, India. Singap. J. Trop. Geogr. 1998, 19, 91–105. [Google Scholar] [CrossRef]
- Verma, R.; Singh, S.; Raj, K.G. Assessment of changes in water-hyacinth coverage of water bodies in northern part of Bangalore city using temporal remote sensing data. Curr. Sci. 2003, 84, 795–804. [Google Scholar]
- Asmare, E. Current Trend of Water Hyacinth Expansion and Its Consequence on the Fisheries around North Eastern Part of Lake Tana, Ethiopia. J. Biodivers. Endanger. Species 2017, 5, 5. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Kibret, A.A.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Dagnew, D.; Abebe, W.B.; Melesse, A.M. Potential of Water Hyacinth Infestation on Lake Tana, Ethiopia: A Prediction Using a GIS-Based Multi-Criteria Technique. Water 2019, 11, 1921. [Google Scholar] [CrossRef] [Green Version]
- Dersseh, M.G.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Abebe, W.B.; Mihret, D.A.; Melesse, A.M. Spatial and Temporal Dynamics of Water Hyacinth and Its Linkage with Lake-Level Fluctuation: Lake Tana, a Sub-Humid Region of the Ethiopian Highlands. Water 2020, 12, 1435. [Google Scholar] [CrossRef]
- Teshome, G.; Getahun, A.; Mengist, M.; Hailu, B. Some biological aspects of spawning migratory Labeobarbus species in some tributary rivers of Lake Tana, Ethiopia. Int. J. Fish. Aquat. Stud. 2015, 3, 136–141. [Google Scholar]
- Gezie, A.; Assefa, W.W.; Getnet, B.; Anteneh, W.; Dejen, E.; Mereta, S.T. Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Boil. Invasions 2018, 20, 2517–2534. [Google Scholar] [CrossRef]
- Conway, D. The Climate and Hydrology of the Upper Blue Nile River. Geogr. J. 2000, 166, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Dile, Y.T.; Tekleab, S.; Ayana, E.K.; Gebrehiwot, S.G.; Worqlul, A.W.; Bayabil, H.K.; Yimam, Y.T.; Tilahun, S.A.; Daggupati, P.; Karlberg, L.; et al. Advances in water resources research in the Upper Blue Nile basin and the way forward: A review. J. Hydrol. 2018, 560, 407–423. [Google Scholar] [CrossRef]
- Vijverberg, J.; Sibbing, F.A.; Dejen, E. Lake Tana: Source of the Blue Nile. In The Nile; Springer: Dordrecht, The Netherlands, 2009; pp. 163–192. [Google Scholar] [CrossRef]
- Ligdi, E.E.; El Kahloun, M.; Meire, P. Ecohydrological status of Lake Tana—A shallow highland lake in the Blue Nile (Abbay) basin in Ethiopia: Review. Ecohydrol. Hydrobiol. 2010, 10, 109–122. [Google Scholar] [CrossRef]
- Zur Heide, F. Feasibility Study for a Lake Tana Biosphere Reserve, Ethiopia. Bundesamt für Naturschutz, BfN. 2012. Available online: https://en.nabu.de/imperia/md/images/nabude/projekteaktionen/international/aethiopien/nabu-f-zur-heide-feasability_study.pdf (accessed on 20 August 2020).
- Wale, A.; Rientjes, T.H.M.; Gieske, A.S.; Getachew, H.A. Ungauged catchment contributions to Lake Tana’s water balance. Hydrol. Process. 2009, 23, 3682–3693. [Google Scholar] [CrossRef]
- Kebede, S.; Travi, Y.; Alemayehu, T.; Marc, V. Water balance of Lake Tana and its sensitivity to fluctuations in rainfall, Blue Nile basin, Ethiopia. J. Hydrol. 2006, 316, 233–247. [Google Scholar] [CrossRef]
- Ayana, E.K.; Worqlul, A.W.; Steenhuis, T.S. Evaluation of stream water quality data generated from MODIS images in modeling total suspended solid emission to a freshwater lake. Sci. Total Environ. 2015, 523, 170–177. [Google Scholar] [CrossRef]
- Zimale, F.A.; Moges, M.A.; Alemu, M.L.; Ayana, E.K.; Demissie, S.S.; Tilahun, S.A.; Steenhuis, T.S. Budgeting suspended sediment fluxes in tropical monsoonal watersheds with limited data: The Lake Tana basin. J. Hydrol. Hydromech. 2018, 66, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Rientjes, T.H.; Perera, B.U.J.; Haile, A.T.; Reggiani, P.; Muthuwatta, L.P. Regionalisation for lake level simulation—The case of Lake Tana in the Upper Blue Nile, Ethiopia. Hydrol. Earth Syst. Sci. 2011, 15, 1167–1183. [Google Scholar] [CrossRef]
- SMEC, I. Hydrological Study of The Tana-Beles Sub-Basins. “part 1.”. Sub-Basins Groundw. Investig. Rep. 2007, 5089018. [Google Scholar]
- Yang, C.; Everitt, J.H. Mapping three invasive weeds using airborne hyperspectral imagery. Ecol. Inform. 2010, 5, 429–439. [Google Scholar] [CrossRef]
- Underwood, E.C.; Mulitsch, M.J.; Greenberg, J.; Whiting, M.L.; Ustin, S.L.; Kefauver, S.C. Mapping Invasive Aquatic Vegetation in the Sacramento-San Joaquin Delta using Hyperspectral Imagery. Environ. Monit. Assess. 2006, 121, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Altena, B.; Mousivand, A.; Mascaro, J.; Kääb, A. Potential and limitations of photometric reconstruction through a flock of dove cubesats. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2017, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Planet Team. Planet Application Program Interface: In Space for Life on Earth. Available online: https://www.planet.com (accessed on 20 August 2020).
- Planet Labs. Planet Imagery Product Specifications. Available online: https://www.planet.com (accessed on 20 August 2020).
- McCabe, M.F.; Aragon, B.; Houborg, R.; Mascaro, J. CubeSats in Hydrology: Ultrahigh-Resolution Insights into Vegetation Dynamics and Terrestrial Evaporation. Water Resour. Res. 2017, 53, 10017–10024. [Google Scholar] [CrossRef] [Green Version]
- Dobrinić, D.; Gašparović, M.; Župan, R. Horizontal Accuracy Assessment of PlanetScope, RapidEye and WorldView-2 Satellite Imagery. In Proceedings of the 18th International Multidisciplinary Scientific Geoconference SGEM 2018, Albena, Bulgaria, 30 June–9 July 2018. [Google Scholar]
- Hang, N.T.T.; Hoa, N.T.; Son, T.P.H.; Nguyen-Ngoc, L. Vegetation Biomass of Sargassum Meadows in An Chan Coastal Waters, Phu Yen Province, Vietnam Derived from PlanetScope Image. J. Environ. Sci. Eng. B 2019, 8, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.A.; Richards, J. Remote Sensing Digital Image Analysis; Springer: Berlin/Heidelberg, Germany, 1999; Volume 3. [Google Scholar]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S. Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresour. Technol. 2018, 251, 390–402. [Google Scholar] [CrossRef]
- Bowman, W.D.; Theodose, T.A.; Schardt, J.C.; Conant, R.T. Constraints of Nutrient Availability on Primary Production in Two Alpine Tundra Communities. Ecology 1993, 74, 2085–2097. [Google Scholar] [CrossRef] [Green Version]
- Santamaría, L. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 2002, 23, 137–154. [Google Scholar] [CrossRef]
- A Davis, M.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Pietrangeli, S. Bathymetry of Lake Tana; Unpublished Report; Studio Pietrangeli: Rome, Italy, 1998. [Google Scholar]
- Ayana, E.K. Validation of Radar Altimetry Lake Level Data and It’s Application in Water Resource Management. ITC. 2007. Available online: https://webapps.itc.utwente.nl/librarywww/papers_2007/msc/wrem/kaba.pdf (accessed on 20 September 2019).
- Carr, G.M.; Duthie, H.C.; Taylor, W.D. Models of aquatic plant productivity: A review of the factors that influence growth. Aquat. Bot. 1997, 59, 195–215. [Google Scholar] [CrossRef]
- Belding, D.L. Water Temperature and Fish Life. Trans. Am. Fish. Soc. 1928, 58, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate Impacts on Agriculture: Implications for Crop Production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Imaoka, T.; Teranishi, S. Rates of nutrient uptake and growth of the water hyacinth [Eichhornia crassipes (mart.) Solms]. Water Res. 1988, 22, 943–951. [Google Scholar] [CrossRef]
- Kasselmann, C. Aquarienpflanzen; Ulmer, E., Ed.; Eugen Ulmer: Stuttgart, Germany, 1995; ISBN 978-3-8186-0699-2. Available online: https://www.ulmer.de/usd-1557211/aquarienpflanzen-.html (accessed on 10 October 2019).
- Zhang, G.; Yao, T.; Xie, H.; Qin, J.; Ye, Q.; Dai, Y.; Guo, R. Estimating surface temperature changes of lakes in the Tibetan Plateau using MODIS LST data. J. Geophys. Res. Atmos. 2014, 119, 8552–8567. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Yang, K.; Yu, Z.; Zhu, Y. Spatiotemporal Variations in Dianchi Lake’s Surface Water Temperature From 2001 to 2017 Under the Influence of Climate Warming. IEEE Access 2019, 7, 115378–115387. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Z.; Luo, Y.; Zhou, X.; Shang, C. Spatial-Temporal Variation of Lake Surface Water Temperature and its Driving Factors in Yunnan-Guizhou Plateau. Water Resour. Res. 2019, 55, 4688–4703. [Google Scholar] [CrossRef]
- Mildrexler, D.J.; Zhao, M.; Running, S.W. A global comparison between station air temperatures and MODIS land surface temperatures reveals the cooling role of forests. J. Geophys. Res. Space Phys. 2011, 116, 116. [Google Scholar] [CrossRef]
- Ayana, E.K.; Zimale, F.A.; Collick, A.S.; Tilahun, S.A.; Elkamil, M.; Philpot, W.D.; Steenhuis, T.S. Monitoring State of Biomass Recovery in the Blue Nile Basin Using Image-Based Disturbance Index. In Nile River Basin; Springer: Berlin/Heidelberg, Germany, 2014; pp. 237–252. [Google Scholar] [CrossRef]
- Nemani, R.R.; Running, S.W. Estimation of Regional Surface Resistance to Evapotranspiration from NDVI and Thermal-IR AVHRR Data. J. Appl. Meteorol. 1989, 28, 276–284. [Google Scholar] [CrossRef]
- Birkett, C.; Reynolds, C.; Beckley, B.; Doorn, B. From research to operations: The USDA global reservoir and lake monitor. In Coastal Altimetry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–50. ISBN 978-3-642-12796-0. [Google Scholar]
- Lloyd, D.S. Turbidity as a Water Quality Standard for Salmonid Habitats in Alaska. N. Am. J. Fish. Manag. 1987, 7, 34–45. [Google Scholar] [CrossRef]
- Funge-Smith, S.; Briggs, M.R. Nutrient budgets in intensive shrimp ponds: Implications for sustainability. Aquaculture 1998, 164, 117–133. [Google Scholar] [CrossRef]
- Fraser, R.N. Hyperspectral remote sensing of turbidity and chlorophyll a among Nebraska Sand Hills lakes. Int. J. Remote Sens. 1998, 19, 1579–1589. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, C.; Muller-Karger, F. Monitoring turbidity in Tampa Bay using MODIS/Aqua 250-m imagery. Remote Sens. Environ. 2007, 109, 207–220. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.A.; Rundquist, D.C.; Leavitt, B.; Barrow, T.; Holz, J.C. Assessing the potential of SeaWiFS and MODIS for estimating chlorophyll concentration in turbid productive waters using red and near-infrared bands. Remote Sens. Environ. 2005, 96, 176–187. [Google Scholar] [CrossRef]
- Quang, N.H.; Sasaki, J.; Higa, H.; Huan, N.H. Spatiotemporal Variation of Turbidity Based on Landsat 8 OLI in Cam Ranh Bay and Thuy Trieu Lagoon, Vietnam. Water 2017, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Dogliotti, A.; Ruddick, K.; Nechad, B.; Doxaran, D.; Knaeps, E. A single algorithm to retrieve turbidity from remotely-sensed data in all coastal and estuarine waters. Remote Sens. Environ. 2015, 156, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Kaba, E.; Philpot, W.D.; Steenhuis, T. Evaluating suitability of MODIS-Terra images for reproducing historic sediment concentrations in water bodies: Lake Tana, Ethiopia. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 286–297. [Google Scholar] [CrossRef]
- Wale, A.; Rientjes, T.; Dost, R.; Gieske, A. Hydrological Balance of Lake Tana Upper Blue Nile Basin, Ethiopia. Neth. ITC 2008. Available online: https://webapps.itc.utwente.nl/librarywww/papers_2008/msc/wrem/wale.pdf (accessed on 10 October 2019).
- Marshall, M.H.; Lamb, H.F.; Huws, D.; Davies, S.J.; Bates, C.R.; Bloemendal, J.; Boyle, J.; Leng, M.J.; Umer, M.; Bryant, C. Late Pleistocene and Holocene drought events at Lake Tana, the source of the Blue Nile. Glob. Planet. Chang. 2011, 78, 147–161. [Google Scholar] [CrossRef]
- Myre, E.; Shaw, R. The turbidity tube: Simple and accurate measurement of turbidity in the field. Mich. Technol. Univ. 2006. Available online: http://serresconseil.com/WASH/Watsanmissionassistant/mainSpace/files/Turbidity-Myre_Shaw.pdf (accessed on 12 August 2019).
- Anteneh, W.; Dejen, E.; Getahun, A. Shesher and Welala Floodplain Wetlands (Lake Tana, Ethiopia): Are They Important Breeding Habitats forClarias gariepinusand the MigratoryLabeobarbusFish Species? Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Nyssen, J.; Moges, M.M.; Enku, T.; Zimale, F.A.; Tilahun, S.A.; Adgo, E.; Steenhuis, T.S. Long-Term Landscape Changes in the Lake Tana Basin as Evidenced by Delta Development and Floodplain Aggradation in Ethiopia. Land Degrad. Dev. 2017, 28, 1820–1830. [Google Scholar] [CrossRef]
- Crétaux, J.-F.; Jelinski, W.; Calmant, S.; Kouraev, A.V.; Vuglinski, V.; Bergé-Nguyen, M.; Gennero, M.-C.; Nino, F.; Del Rio, R.A.; Cazenave, A.; et al. SOLS: A lake database to monitor in the Near Real Time water level and storage variations from remote sensing data. Adv. Space Res. 2011, 47, 1497–1507. [Google Scholar] [CrossRef]
- Birkett, C.M.; Beckley, B. Investigating the performance of the Jason-2/OSTM radar altimeter over lakes and reservoirs. Mar. Geod. 2010, 33, 204–238. [Google Scholar] [CrossRef]
- Puhe, J. Growth and development of the root system of Norway spruce (Picea abies) in forest stands—A review. For. Ecol. Manag. 2003, 175, 253–273. [Google Scholar] [CrossRef]
- Santiago, C.M., Jr. Some environmental factors affecting growth of water hyacinth (Eichhornia crassipes (Mart.) Solms). Philipp. J. Sci. (Philippines) 1984, 113, 67–82. [Google Scholar]
- El-Gendy, A.; Biswas, N.; Bewtra, J. Growth of Water Hyacinth in Municipal Landfill Leachate with Different pH. Environ. Technol. 2004, 25, 833–840. [Google Scholar] [CrossRef]














© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worqlul, A.W.; Ayana, E.K.; Dile, Y.T.; Moges, M.A.; Dersseh, M.G.; Tegegne, G.; Kibret, S. Spatiotemporal Dynamics and Environmental Controlling Factors of the Lake Tana Water Hyacinth in Ethiopia. Remote Sens. 2020, 12, 2706. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12172706
Worqlul AW, Ayana EK, Dile YT, Moges MA, Dersseh MG, Tegegne G, Kibret S. Spatiotemporal Dynamics and Environmental Controlling Factors of the Lake Tana Water Hyacinth in Ethiopia. Remote Sensing. 2020; 12(17):2706. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12172706
Chicago/Turabian StyleWorqlul, Abeyou W., Essayas K. Ayana, Yihun T. Dile, Mamaru A. Moges, Minychl G. Dersseh, Getachew Tegegne, and Solomon Kibret. 2020. "Spatiotemporal Dynamics and Environmental Controlling Factors of the Lake Tana Water Hyacinth in Ethiopia" Remote Sensing 12, no. 17: 2706. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12172706