A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems
Abstract
:1. Introduction
2. Methods
2.1. Study Sites
2.2. Data Collection
2.3. Data Processing
2.4. Penguin Count Data
2.5. Population Count Workflow
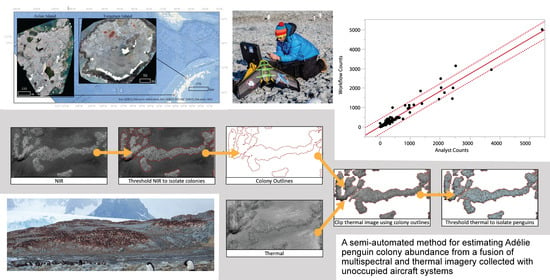
2.5.1. Step 1. Multispectral Colony Delineation
2.5.2. Steps 2 and 3. Thermal Colony Assessment
2.6. Comparison with Manual Imagery Counts
3. Results
3.1. Manual and Automated Counts
3.2. Area-Based Assessments
3.3. Manual vs. Automated Counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agnew, D.J. Review—The CCAMLR Ecosystem Monitoring Programme. Antarct. Sci. 1997, 9. [Google Scholar] [CrossRef]
- Lancia, R.A.; Kendall, W.L.; Pollock, K.H.; Nichols, J.D. Estimating the number of animals in wildlife populations. In Techniques for Wildlife Investigations and Management; Braun, C.E., Ed.; Wildlife Society: Bethesda, MD, USA, 2005; pp. 106–153. [Google Scholar]
- Sasse, D.B. Job-Related Mortality of Wildlife Workers in the United States, 1937–2000. Wildl. Soc. Bull. 2003, 31, 1015–1020. [Google Scholar]
- Trathan, P.N. Image analysis of color aerial photography to estimate penguin population size. Wildl. Soc. Bull. 2004, 32, 332–343. [Google Scholar] [CrossRef]
- Fraser, W.R.; Carlson, J.C.; Duley, P.A.; Holm, E.J.; Patterson, D.L. Using Kite-Based Aerial Photography for Conducting Adélie Penguin Censuses in Antarctica. Waterbirds Int. J. Waterbird Biol. 1999, 22, 435. [Google Scholar] [CrossRef]
- Montaigne, F. Fraser’s Penguins: A Journey to the Future in Antarctica, 1st ed.; Henry Holt and Co.: New York, NY, USA, 2010; ISBN 978-0-8050-7942-5. [Google Scholar]
- Stonehouse, B. Introdcution: The Spheniscidae. In The Biology of Penguins; The Macmillan Press: London, UK, 1975; pp. 1–15. [Google Scholar]
- Waluda, C.M.; Dunn, M.J.; Curtis, M.L.; Fretwell, P.T. Assessing penguin colony size and distribution using digital mapping and satellite remote sensing. Polar Biol. 2014, 37, 1849–1855. [Google Scholar] [CrossRef]
- Hollings, T.; Burgman, M.; van Andel, M.; Gilbert, M.; Robinson, T.; Robinson, A. How do you find the green sheep? A critical review of the use of remotely sensed imagery to detect and count animals. Methods Ecol. Evol. 2018, 9, 881–892. [Google Scholar] [CrossRef]
- Southwell, C.; McKinlay, J.; Low, M.; Wilson, D.; Newbery, K.; Lieser, J.L.; Emmerson, L. New methods and technologies for regional-scale abundance estimation of land-breeding marine animals: Application to Adélie penguin populations in East Antarctica. Polar Biol. 2013, 36, 843–856. [Google Scholar] [CrossRef]
- Pfeifer, C.; Barbosa, A.; Mustafa, O.; Peter, H.U.; Rümmler, M.C.; Brenning, A. Using fixed-wing uav for detecting and mapping the distribution and abundance of penguins on the South Shetlands Islands, Antarctica. Drones 2019, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, O.; Braun, C.; Esefeld, J.; Knetsch, S.; Maercker, J.; Pfeifer, C.; Rümmler, M.C. Detecting Antarctic Seals And Flying Seabirds by UAV. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 4, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Laliberte, A.S.; Ripple, W.J. Automated Wildlife Counts from Remotely Sensed Imagery. Wildl. Soc. Bull. 2003, 31, 362–371. [Google Scholar]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Bajzak, D.; Piatt, J.F. Computer-Aided Procedure for Counting Waterfowl on Aerial Photographs. Wildl. Soc. Bull. 1990, 18, 125–129. [Google Scholar]
- Gilmer, D.S.; Brass, J.A.; Strong, L.L.; Card, D.H. Goose Counts from Aerial Photographs Using an Optical Digitizer. Wildl. Soc. Bull. 1988, 16, 204–206. [Google Scholar]
- Chabot, D.; Francis, C.M. Computer-automated bird detection and counts in high-resolution aerial images: A review. J. Field Ornithol. 2016, 87, 343–359. [Google Scholar] [CrossRef]
- Cunningham, D.J.; Anderson, W.H.; Anthony, R.M. An image-processing program for automated counting. Wildl. Soc. Bull. 1996, 24, 345–346. [Google Scholar]
- Groom, G.; Stjernholm, M.; Nielsen, R.D.; Fleetwood, A.; Petersen, I.K. Remote sensing image data and automated analysis to describe marine bird distributions and abundances. Ecol. Inform. 2013, 14, 2–8. [Google Scholar] [CrossRef]
- Sardà-Palomera, F.; Bota, G.; Viñolo, C.; Pallarés, O.; Sazatornil, V.; Brotons, L.; Gomáriz, S.; Sardà, F. Fine-scale bird monitoring from light unmanned aircraft systems. IBIS (Lond. 1859) 2012, 154, 177–183. [Google Scholar] [CrossRef]
- Andrew, M.E.; Shephard, J.M. Semi-automated detection of eagle nests: An application of very high-resolution image data and advanced image analyses to wildlife surveys. Remote Sens. Ecol. Conserv. 2017, 3, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Lyons, M.B.; Brandis, K.J.; Murray, N.J.; Wilshire, J.H.; McCann, J.A.; Kingsford, R.T.; Callaghan, C.T. Monitoring large and complex wildlife aggregations with drones. Methods Ecol. Evol. 2019, 10, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- LaRue, M.A.; Lynch, H.J.; Lyver, P.O.B.; Barton, K.; Ainley, D.G.; Pollard, A.; Fraser, W.R.; Ballard, G. A method for estimating colony sizes of Adélie penguins using remote sensing imagery. Polar Biol. 2014, 37, 507–517. [Google Scholar] [CrossRef]
- Witharana, C.; Lynch, H.J. An object-based image analysis approach for detecting penguin guano in very high spatial resolution satellite images. Remote Sens. 2016, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- McNeill, S.; Barton, K.; Lyver, P.; Pairman, D. Semi-automated penguin counting from digital aerial photographs. In Proceedings of the 2011 IEEE International Geoscience and Remote Sensing Symposium, Vancouver, BC, Canada, 24–29 July 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 4312–4315. [Google Scholar]
- Johnston, D.W. Unoccupied Aircraft Systems in Marine Science and Conservation. Ann. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahawi, R.A.; Dandois, J.P.; Holl, K.D.; Nadwodny, D.; Reid, J.L.; Ellis, E.C. Using lightweight unmanned aerial vehicles to monitor tropical forest recovery. Biol. Conserv. 2015, 186, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Mancini, F.; Dubbini, M.; Gattelli, M.; Stecchi, F.; Fabbri, S.; Gabbianelli, G.; Mancini, F.; Dubbini, M.; Gattelli, M.; Stecchi, F.; et al. Using Unmanned Aerial Vehicles (UAV) for High-Resolution Reconstruction of Topography: The Structure from Motion Approach on Coastal Environments. Remote Sens. 2013, 5, 6880–6898. [Google Scholar] [CrossRef] [Green Version]
- Seymour, A.C.; Ridge, J.T.; Rodriguez, A.B.; Newton, E.; Dale, J.; Johnston, D.W. Deploying Fixed Wing Unoccupied Aerial Systems (UAS) for Coastal Morphology Assessment and Management. J. Coast. Res. 2018, 34, 704–717. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Topouzelis, K.; Pavlogeorgatos, G.; Papakonstantinou, A.; Topouzelis, K.; Pavlogeorgatos, G. Coastline Zones Identification and 3D Coastal Mapping Using UAV Spatial Data. ISPRS Int. J. Geo-Inf. 2016, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Seymour, A.C.; Ridge, J.T.; Newton, E.; Rodriguez, A.B.; Johnston, D.W. Geomorphic response of inlet barrier islands to storms. Geomorphology 2019, 339, 127–140. [Google Scholar] [CrossRef]
- Inoue, J.; Curry, J.A. Application of Aerosondes to high-resolution observations of sea surface temperature over Barrow Canyon. Geophys. Res. Lett. 2004, 31, L14312. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.M.; Neilson, B.T.; McKee, M.; Chen, Y. Thermal remote sensing with an autonomous unmanned aerial remote sensing platform for surface stream temperatures. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 5049–5052. [Google Scholar]
- Durban, J.W.; Moore, M.J.; Chiang, G.; Hickmott, L.S.; Bocconcelli, A.; Howes, G.; Bahamonde, P.A.; Perryman, W.L.; LeRoi, D.J. Photogrammetry of blue whales with an unmanned hexacopter. Mar. Mammal Sci. 2016, 32, 1510–1515. [Google Scholar] [CrossRef]
- Christiansen, F.; Dawson, S.; Durban, J.; Fearnbach, H.; Miller, C.; Bejder, L.; Uhart, M.; Sironi, M.; Corkeron, P.; Rayment, W.; et al. Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar. Ecol. Prog. Ser. 2020. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mamm. Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Jones, G.P.; Pearlstine, L.G.; Percival, H.F. An Assessment of Small Unmanned Aerial Vehicles for Wildlife Research. Wildl. Soc. Bull. 2006, 34, 750–758. [Google Scholar] [CrossRef]
- Watts, A.C.; Perry, J.H.; Smith, S.E.; Burgess, M.A.; Wilkinson, B.E. Szantoi Small Unmanned Aircraft Systems for Low-Altitude Aerial Surveys. J. Wildlife Manag. 2010, 74, 1614–1619. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Christiansen, P.; Steen, K.; Jørgensen, R.; Karstoft, H. Automated Detection and Recognition of Wildlife Using Thermal Cameras. Sensors 2014, 14, 13778–13793. [Google Scholar] [CrossRef]
- Gillette, G.L.; Coates, P.S.; Petersen, S.; Romero, J.P. Can reliable sage-grouse lek counts be obtained using aerial infrared technology? J. Fish Wildl. Manag. 2013, 4, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Gillette, G.L.; Reese, K.P.; Connelly, J.W.; Colt, C.J.; Knetter, J.M. Evaluating the potential of aerial infrared as a lek count method for prairie grouse. J. Fish Wildl. Manag. 2015, 6, 486–497. [Google Scholar] [CrossRef]
- McCafferty, D.J. Applications of thermal imaging in avian science. IBIS (Lond. 1859) 2013, 155, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Burn, D.M.; Webber, M.A.; Udevitz, M.S. Application of Airborne Thermal Imagery to Surveys of Pacific Walrus. Wildl. Soc. Bull. 2006, 34, 51–58. [Google Scholar] [CrossRef]
- Gonzalez, L.; Montes, G.; Puig, E.; Johnson, S.; Mengersen, K.; Gaston, K.; Gonzalez, L.F.; Montes, G.A.; Puig, E.; Johnson, S.; et al. Unmanned Aerial Vehicles (UAVs) and Artificial Intelligence Revolutionizing Wildlife Monitoring and Conservation. Sensors 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrétien, L.-P.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Lhoest, S.; Linchant, J.; Quevauvillers, S.; Vermeulen, C.; Lejeune, P. How Many Hippos (Homhip): Algorithm For Automatic Counts Of Animals With Infra-Red Thermal Imagery From UAV. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2015, 40. [Google Scholar] [CrossRef] [Green Version]
- Seymour, A.C.; Dale, J.; Hammill, M.; Halpin, P.N.; Johnston, D.W. Automated detection and enumeration of marine wildlife using unmanned aircraft systems ({UAS}) and thermal imagery. Sci. Rep. 2017, 7, 45127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.; Schoonmaker, J.; Saggese, S. Automated detection of marine animals using multispectral imaging. In Proceedings of the 2014 Oceans-St. John’s, OCEANS 2014, St. John’s, NL, Canada, 14–19 September 2014; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2015. [Google Scholar]
- DiGiacomo, A.E.; Bird, C.N.; Pan, V.G.; Dobroski, K.; Atkins-Davis, C.; Johnston, D.W.; Ridge, J.T. Modeling Salt Marsh Vegetation Height Using Unoccupied Aircraft Systems and Structure from Motion. Remote Sens. 2020, 12, 2333. [Google Scholar] [CrossRef]
- Arona, L.; Dale, J.; Heaslip, S.G.; Hammill, M.O.; Johnston, D.W. Assessing the disturbance potential of small unoccupied aircraft systems (UAS) on gray seals (Halichoerus grypus) at breeding colonies in Nova Scotia, Canada. PeerJ 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Franzini, M.; Ronchetti, G.; Sona, G.; Casella, V. Geometric and Radiometric Consistency of Parrot Sequoia Multispectral Imagery for Precision Agriculture Applications. Appl. Sci. 2019, 9, 5314. [Google Scholar] [CrossRef] [Green Version]
- ESRI ArcGIS Desktop. Release 10.5.1; Environmental Systems Research Institute: Redlands, CA, USA, 2017. [Google Scholar]
- iTAG-Photo Tagging Software, version 0.7; iTAG: Sydney, Australia, 2015; Available online: https://www.itagsoftware.com/ (accessed on 11 November 2020).
- Ozyavuz, M.; Bilgili, B.C.; Salici, A. Determination of vegetation changes with NDVI method. J. Environ. Prot. Ecol. 2015, 16, 264–273. [Google Scholar]
- Zhu, R.; Liu, Y.; Ma, E.; Sun, J.; Xu, H.; Sun, L. Nutrient compositions and potential greenhouse gas production in penguin guano, ornithogenic soils and seal colony soils in coastal {Antarctica}. Antarct. Sci. 2009, 21, 427–438. [Google Scholar] [CrossRef]
- Almendingen, K.; Meltzer, H.; Pedersen, J.; Nilsen, B.; Ellekjær, M. Near infrared spectroscopy—a potentially useful method for rapid determination of fat and protein content in homogenized diets. Eur. J. Clin. Nutr. 2000, 54, 20–23. [Google Scholar] [CrossRef]
- Rees, W.G.; Brown, J.A.; Fretwell, P.T.; Trathan, P.N. What colour is penguin guano? Antarct. Sci. 2017, 29, 417–425. [Google Scholar] [CrossRef]




| Study Site | RGB (cm) | Multispectral (cm) | Thermal (cm) |
|---|---|---|---|
| Avian | 2.59 | 6.84 | 15.2 |
| Torgersen | 2.28 | 7.58 | 11.6 |
| Avian | Torgersen | |||||||
|---|---|---|---|---|---|---|---|---|
| Analyst 1 | Analyst 2 | Mean | SD | Analyst 1 | Analyst 2 | Mean | SD | |
| Adult | 29,392 | 25,498 | 27,445 | 2753.4 | 984 | 606 | 795 | 267.2 |
| Juvenile | 18,108 | 25,967 | 22,038 | 5557.1 | 199 | 589 | 394 | 275.7 |
| Total | 47,500 | 51,465 | 49,483 | 2803.6 | 1183 | 1195 | 1189 | 8.5 |
| RGB | Thermal | NIR Reflectance | |||
| Analyst Avg. | Total | Guano (sq.m) | Total (1.27 peng/sq.m) | Total (2 peng/sq.m) | |
| Avian | 49,483 | 47,525 | 38,318.75 | 48,665 | 76,637 |
| RGB | Thermal | NDVI | |||
| Analyst Avg. | Total | Guano (sq.m) | Total (0.90 peng/sq.m) | Total (2 peng/sq.m) | |
| Torgersen | 1189 | 1401 | 1439.3 | 1295 | 2879 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bird, C.N.; Dawn, A.H.; Dale, J.; Johnston, D.W. A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems. Remote Sens. 2020, 12, 3692. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223692
Bird CN, Dawn AH, Dale J, Johnston DW. A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems. Remote Sensing. 2020; 12(22):3692. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223692
Chicago/Turabian StyleBird, Clara N., Allison H. Dawn, Julian Dale, and David W. Johnston. 2020. "A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems" Remote Sensing 12, no. 22: 3692. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223692