1. Introduction
One of the main problems currently affecting water resources is the eutrophication of water bodies, both coastal and freshwater [
1,
2]; it was already known ten years ago that around 20% of European lakes were suffering from nutrient enrichment [
3]. In the European Union, the management of reservoirs and natural waterbodies is directly linked to the Water Framework Directive [
4], which requires periodic monitoring; this being a common trait all over the world [
5]. Most of the reservoir monitoring programs are currently based on measurements taken in the field making it difficult to capture the spatial and temporal variability of phenomena such as algal blooms, with irregular spatiotemporal patterns and potential toxicity (Cyanobacterial blooms). This circumstance hampers the possibility of taking timely and informed management decisions to prevent, counteract or mitigate the negative effects of eutrophication in drinking water reservoirs where “early warning” systems are increasingly needed.
The application of remote sensing for the measurement of a range of parameters indicative of water quality (temperature, transparency, turbidity, concentration of photosynthetic pigments, colored dissolved organic matter, etc.) has been proven effective in capturing this variability in aquatic ecosystems through the use of satellite images from multispectral sensors such as a Medium Resolution Imaging Spectrometer (MERIS), the various Landsat Thematic Mapper/Enhanced Thematic Mapper (TM/ETM+), Operational Land Imager (OLI) sensors, or more recently Sentinel 2 MSI (e.g., [
6,
7,
8,
9,
10,
11,
12,
13,
14]).
The retrieval of phytoplankton chlorophyll-a concentration (chl-a) from remotely sensed data is one of the key issues in aquatic remote sensing. The spectral signature of chl-a is characterized by strong absorption in the blue (443 nm) and red wavelengths (near 675 nm) and high reflectance in the green (550–555 nm) and rededge spectrum regions (685–710 nm) [
15,
16,
17]. These spectral features have been used to develop several band ratios to quantify chl-a concentration in inland and near-coastal transitional waters through empirical approaches, providing timely and accurate information [
9]. Specifically, empirical algorithms based on green and blue bands have been successfully applied to open oceans (Case 1 waters [
18]) and oligotrophic inland waters [
19,
20], while the near-infrared (NIR) to red reflectance ratios are used in productive waters (Case 2). In the latter, chl-a is not correlated with the other optically active constituents, and the absorption by Coloured Dissolved Organic Matter (CDOM), tripton, and other pigments overlaps the absorption peak of chl-a in the blue spectral region. In this type of water, the only spectral range maximally sensitive to chl-a is the Red-NIR, which is still affected by the residual absorption by CDOM and tripton. Several algorithms have been developed using this spectral range, all based on the properties of the reflectance peak near 700 nm, which is maximally sensitive to the variations in chl-a in water and its relationships with the red chl-a absorption band (660–690 nm). Still, the backscattering by all particulate matter is also affecting the reflectance in this spectral region, hence a third band in the NIR region (>740 nm) was added by [
21] to account for this effect in chl-a estimation, as the variations in particulate backscattering, control variations in reflectance in this region [
21,
22,
23]. The use of combined algorithms has been also suggested for the monitoring of these different water types and trophic states [
24].
Two of the main limitations that affect the systematic or periodic monitoring of aquatic ecosystems through satellite remote sensing are: on the one hand, the presence of atmospheric effects, which can leave the managers of water bodies without information for extended time periods, and on the other hand, the spatial resolution of sensors on board satellites. Multispectral sensors designed for water applications are mainly designed for ocean water monitoring (e.g., MODerate resolution Imaging Spectroradiometer (MODIS), Medium Resolution Imaging Spectrometer (MERIS) or Ocean and Land Colour Instrument (OLCI)) and their coarse spatial resolution is lower than that required to monitor small systems. It is for this reason that the sensors currently employed for the monitoring of most inland water bodies are sensors initially designed for terrestrial applications as Sentinel 2 MultiSpectral Instrument (MSI) and Landsat 8 Operational Land Imager (OLI).
As with the multispectral sensors on board satellites, the commercial technology currently available for sensors that can be boarded on UAVs is not specifically designed for aquatic but for terrestrial applications, mostly for precision agriculture. Hence, the design of the sensor spectral bands is not ideal, but theoretically they can be used for some aquatic applications, including the quantification of the pigment chl-a.
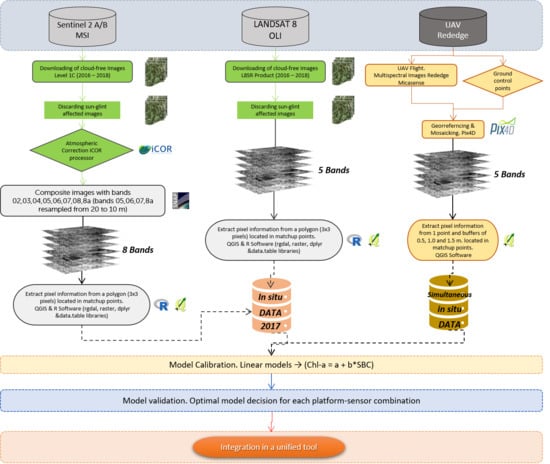
Based on this background, we propose a methodology for the monitoring of aquatic resources trying to maximize the periodicity of data acquisition and gap-filling by combining sensors and platforms, complementing an established monitoring program. On it, freely distributed images obtained through multispectral sensors on board two satellite platforms (Sentinel 2 and Landsat 8), used as a virtual constellation [
25,
26], are combined with commercial multispectral sensors mounted on UAV platforms. This approach is seeking to combine the affordability, stability, quality and continuity of ESA and NASA missions with the flexibility and spatial resolution provided by the use of UAV platforms, to make the most of these resources in favor of efficient management of aquatic resources. The empirical approach has been used to test the retrieval of chl-a as a quantitative descriptor of the presence of phytoplankton and the degree of eutrophication of a small water body, using images at different scales taken with three multispectral sensors with different characteristics, in a test carried out during 2017 and 2018 in a drinking water reservoir regularly affected by the presence of cyanobacteria.
The objectives of the study are: (1) To evaluate the performance of different algorithms and band indices in a small waterbody, by using images obtained with three different sensors, during 2016, 2017 and 2018. (2) To validate the application of free-distributed multispectral images from Sentinel 2 and Landsat 8 satellites to improve the continuous monitoring of small reservoirs, in an area of very high cloud cover (Galicia, NW Iberian Peninsula). (3) To assess the detection and quantification capability for chl-a of a remote sensing system consisting of a commercial multispectral camera on board a multirotor UAV and to verify its potential application in the absence of valid satellite images.
4. Discussion
Phytoplankton blooms can be highly variable in space and time, cyanobacterial blooms being especially complex due to their buoyancy capacity that enables them to move vertically along the water column [
52]. These blooms present a spatial patchy distribution and a fast development of even only some hours [
53]. Therefore, their detection and monitoring are difficult tasks that cannot be tackled only by in situ point measurements; the synoptic view offered by satellites and UAV multispectral or hyperspectral imagery is an excellent complement to this routine type of sampling [
14,
54]
The data provided by Sentinel 2 and Landsat 8, when combined in the form of a virtual constellation, opened up new opportunities for capturing limnological dynamics that reflect rates of change at an unprecedent resolution [
26,
36,
54]. The Landsat 8 equatorial repeat cycle (since 2013) is 16 days, while each of the Sentinels 2 is 10 days, which becomes 5 days when the two are combined (Sentinel 2A since 2015 and S2B since 2017). The revisit time of the virtual constellation formed by the Landsat 8 and Sentinel 2 satellites has been estimated at ~2.9 days [
25], which can be even lower in waterbodies located in latitudes where two overpasses of any of the satellites overlap. Not only the temporal but also the spatial resolution of these satellites (10 m. in case of Sentinel 2 MSI and 30 m. in case of Landsat 8 OLI) has broadened the scope of the inland water quality monitoring through satellite imagery, making it possible to capture much smaller waterbodies than the ones usually studied with dedicated multispectral ocean color sensors on board other satellites (e.g., MERIS or Sentinel 3 OLCI with a 300-m. resolution).
One way to make the most of this combination of platforms and sensors is to validate which models or combination of models are suitable for each type of sensor and type of waterbody and combine the results into a single multi-platform tool with medium to high spatial resolution (10–30 m), which will raise to a very high resolution (8 cm.) in the events of UAV imagery availability. As a pilot experience, we studied a very small waterbody, focusing the efforts on the retrieval of chl-a concentration as a proxy for phytoplankton biomass and trophic status.
The inference process followed in Remote Sensing to obtain information about objects from measurements made from space is always less than perfect. The development of remote sensing products to quantify biophysical parameters is subject to different types and degree of uncertainties due to different sources of error inside of the processing chain. Based on the Image Chain Approach to identify uncertainty in Remote Sensing [
55,
56], every step in the processing chain from the capture of the image by the sensor to the final product has an associated uncertainty. This uncertainties propagate along the entire chain, but we will focus the analysis of the uncertainties, in our proposed processing scheme, in the weakest points identified, which also have the potential to be improved in the framework of any established monitoring program: the selection of the time window for the match-up points and the performance of the applied atmospheric correction.
The first source of uncertainty is related to the time lapse (± 3 days) between the image acquisition and the in situ ground truth data. Loew et al. [
57], in their description of uncertainties in the validation process, state that, regarding the uncertainty due to imperfect spatiotemporal collocation, a compromise should be achieved among two concerns: on the one hand, the measurements should be close to each other relative to the spatiotemporal scale on which the variability of the field becomes comparable to the measurement uncertainties; and on the other hand, the need for sufficient collocated pairs to allow a statistical analysis. In our case, the compromise to minimize the uncertainty of the obtained product had to be achieved among the available data (potential matchups) and the frequency and duration of the algal blooms in the area. Trying to minimize this uncertainty, the data of 2017 were used for algorithm calibration, when the intensity and duration of the algal blooms showed a lower change rate of the 3 studied years (
Table 1). Moreover, the extremely high values in sampling point BP were not used in the calibration and validation exercises, being considered exceptional both in time and space and thus with the higher probabilities of not being captured by the satellite images. However, the valid time difference between in situ sampling and satellite overpass used in our work, similar to that used by [
51,
58,
59], is a source of uncertainty that was taken into account when interpreting the results. The uncertainty regarding spatio-temporal collocation can be easily reduced by achieving a compromise with the official agencies in charge of in situ monitoring in which a prioritization of satellite overpassing dates should be established for in situ sampling.
The second source of higher uncertainty identified was the atmospheric correction algorithm applied for each source of data. Lacking vicarious calibration data simultaneous with the satellite overpass to validate the applied AC, we could not give a quantitative estimate of our uncertainty. Nevertheless, this exercise has been done for the Sentinel 2A MSI sensor by Warren et al. [
60] in a thorough study evaluating six free source processors for the atmospheric correction over coastal and inland waters. In their comparison against above-water optical in situ measurements, none of the processors performed well over the entire band set tested (444–865 nm). The authors quantify the uncertainties related to the AC algorithms through the value of the Mean Absolute Percentage Difference (ψ), finding the that green (560 nm) and blue (490 nm) bands were best handled by all ACs, but the red, red-edge and NIR bands had often very high uncertainties. More precisely, the uncertainties associated with the atmospheric correction performed by iCOR in Bands 4, 5 and 7 (the most useful in our study to compute 2BDA and 3BDA algorithms) were quantified ψ = 106.05%, ψ = 149.00% and ψ = 681.75%, respectively, while the Root Mean Squared Errors (RMSE) were 0.0029, 0.0027 and 0.0033 sr
−1, respectively.
The performance of atmospheric correction applied to MSI data in our study was also evaluated over inland waters by Pereira-Sandoval et al. [
61], showing that other processors were more effective in case of oligotrophic to meso-eutrophic waters, while iCOR showed moderately good results in hypereutrophic environments.
In case of Landsat 8, a similar exercise was performed by Ilori et al. [
62] in which four AC processors’ performances (including the Landsat 8 Surface Reflectance Code (LaSRC)) were tested against in situ data acquired mainly over coastal waters from the OC component of the Aerosol Robotic Network (AERONET-OC) for Bands 1 to 4 of OLI sensor. Even though AERONET-OC sites are not representative of inland waters, their results showed a poor performance of L8SR product in OLI Bands 1 and 2 but a good performance at a few sites located in nearshore and inland waters. In this assessment, Bands 3 and 4 (used in our final chosen algorithms) showed an RMSE of 0.0030 and 0.0022 sr
−1, respectively, which is similar to what was found for Sentinel 2 MSI by Warren et al. [
60] for Sentinel 2 MSI. Even though other valuable comparative exercises have been done regarding the performance and associated uncertainties of the AC processor for OLI data used in our study [
63], they were performed over different land covers and so were not taken as a reference for our work.
Another source of uncertainty regarding AC is the adjacency effect related to the distance to shore. This effect cause that the reflected light field from the neighboring land pixels, which are brighter, gets into the path from the target (water pixel) to the sensor through atmospheric scattering [
64]. This also makes the reflectance value from the water pixels near the coast larger, and, hence, these datasets are prone to errors for the derivation of water quality parameters. This effect has been shown in the analysis made in our study comparing the Rrs reflectance of the outer and central pixels of the reservoir. Our results show how the Rrs reflectance of outer pixels have a higher value in the NIR band (B8 S2 MSI) than the central ones. This high NIR reflectance over inland and coastal waters due to adjacency effects has been already reported based on airborne and spaceborne data [
8]. In the study of Warren et al. [
60], it was also shown that iCOR performed poorly in coastal waters but much better in inland waters, due to the land/based methodologies applied to this processor. This result supports the use of iCOR when adjacency effects can be challenging, as in our case.
In spite of this, not only in our study but in some others using different AC processors [
59,
61] and methodologies [
46], promising results were obtained in retrieving chl-a using red and NIR band combinations from Sentinel 2 MSI data. Further developments in the correction of these bands and the specific correction of the adjacency effects, which is only built-in in iCOR of all the publicly available AC processors, it is expected to improve the retrieval of chl-a in inland waters and reduce the associated uncertainties.
The propagation of these errors until the product output is leading to the uncertainties found in our product validation phase. The uncertainty associated with the final output of our Remote Sensing chain was evaluated for every model with different pairwise metrics describing bias and accuracy, as is discussed below.
The development of algorithms for Landsat 8 OLI, with a spatial resolution of 30 m., is hampered by the small size of the reservoir, that makes the adjacency effects an important issue in this case [
14]. Moreover, the need to move the match-up points in the reservoir from the shoreline in order to avoid this effect as much as possible can introduce a source of error in the algorithm development. Nevertheless, almost all the algorithms and Spectral Band Combinations tested were positively and significatively correlated with in situ chl-a values. Both the calibration and validation showed that the most appropriate Spectral Band Combinations in Landsat 8 OLI for the retrieval of chl-a were those based on red and NIR bands, specifically in our study: 2BDA, NDVI and SABI. The SABI is an empirical algorithm developed in order to detect water floating biomass that has an NIR response similar to land vegetation, and also includes blue and green spectral bands [
48].
Although the correlations in the calibration of the models were good, our validation results did not show a good match between the measured and the predicted values (
Figure 5). Other studies did not obtain good results with OLI data when developing algorithms applying 2BDA [
38,
51,
58]. Watanabe et al. [
38] found no relationship with simulated Rrs spectra for OLI/Landsat-8 based on field data in the eutrophic Barra Bonita Reservoir (Brazil), a bigger and deeper waterbody. In this work, also made in a higher chl-a concentration circumstance (mean values ranging from 120.4 to 428.7 µgr/L), the authors suggest that the 2BDA index, as proposed for clear waters, could not explain the chl-a distribution in their reservoir. The best results were obtained in this case with the SLOPE model developed by Mishra and Mishra [
65], which uses red and green bands. Similarly, Boucher et al. [
51] did not find a suitable model using the 2BDA algorithm with Landsat 8 images Level 1 corrected atmospherically with the dark object subtraction (DOS) method. In this study, made on a regional scale, including different lakes in New Hampshire and Maine (US), the best performing models where Kab1, Kab2 and KIVU, all based in blue and green bands. None of them used the L8SR product for the calibration of the models, which is also a methodological difference. This product has been evaluated for aquatic applications by Bernardo et al. [
37]. L8SR was considered by the authors to provide suitable Rrs values for all OLI bands despite achieving the poorest results in Band 5 (NIR). However, our model calibration results with red and NIR bands show that this level 2 product made available by the USGS could be applied successfully for chl-a retrieval even in small waterbodies and should be further investigated with different trophic situations. Not only Red-NIR combinations were found correlated with in situ data in our study, but also algorithms using blue and green bands, as SABI, with very similar values in the validation of statistical metrics, but worst results in the validation graphical output. The worst results were obtained with all algorithms and indices using Band 1 (coastal-aerosol) (Kab2, B3B1 and GB1; cf.
Table 3,
Table 5 and
Figure 4 and
Figure 5). Our results confirm that green and blue band combinations are not the most suitable for retrieving chl-a in complex waters but also that the performance improves when Band 2 is used. This shows the difficulty of performing an accurate atmospheric correction for this ultra-blue band for inland aquatic applications. Wang et al. [
66] also found big errors when computing band ratios with Band 1 data atmospherically corrected with different AC algorithms in their assessment of Landsat-8 OLI atmospheric correction algorithms for inland waters, even though they did not assess the L8SR product. The authors reported the same situation for the band ratios formed by the NIR band with all AC algorithms assessed.
Sentinel 2 data have been extensively tested for chl-a retrieval since its launching in 2015 and 2017, both applying empirical and analytical modelling approaches [
14,
46,
54,
59,
61,
67]. Our results agree with the studies applying empirical modelling approaches [
46,
59,
61], which evaluated the performance of the 3BDA model with good results, even though all of them used what we called here the 3BDA_1 version (with B6 as λ
3). Moreover, in agreement with our study are the results obtained by Xu et al. (2018), who also found good relationships with 3BDA, 2BDA and NDCI, although they used a different formulation of the 2BDA algorithm (B5/B4).
To the best of our knowledge, there is only one other study showing the performance of the commercial sensor Rededge Micasense for chl-a retrieval in waterbodies [
68], but, in this case, the authors do not use Spectral band Combinations based in known spectral features of the chl-a but fit single and multiple variable linear approaches concluding that the best models in their case where those using green and red bands. We can nevertheless consider the results of our study as the first to test the algorithms developed, be proven effective for satellite remote sensing, adapted to Rededge Micasense sensor, and be tested in different trophic conditions. Again, the lack of field radiometry (as also happens in the study of Arango and Nairn [
68]) entails a higher level of uncertainty in the final product. A radiometric calibration made by Cao et al. [
69] for Rededge Micasense, showed in laboratory experiments a good relationship between ASD reflectance and Micasense with R
2 all above 0.96 for all bands. In field experiments, the comparison with the Pix4D processed image showed lower accuracy (overestimation) in the rededege and NIR regions, but a consistency in the spectral signature shape which reduced the uncertainty when using normalized indices.
When analyzing separately each flight data, the most suitable algorithms were again different combinations of red and NIR bands, including a modification of the 3BDA which excludes the last part of the equation. In our case, using the first computation involving λ
1 and λ
2, without removing the effect of the backscattering of the medium with the use of λ
3, gave the best results with this sensor in low chl-a conditions. The optimal position of λ
3 in 2BDA and 3BDA algorithms were established in practice by Dall’Olmo and Gitelson [
70] in 720–740 nm in turbid productive waters. The NIR band of the Rededege Micasense is centered in 840 nm, hence too far from the optimal range, and proved not useful in our study in low chl-a values. As expected by the formulation of the algorithm, when applied in turbid conditions with high chl-a during the second UAV flight, this modification did not perform well (R
2 = 0.4;
Pearson r = 0.6) as the effect of the backscattering of a medium with a high load of suspended particles should be removed with the λ
3 spectral region. Therefore, in this case 2BDA and SABI were the best performing algorithm in the presence of a bloom and high chl-a concentration. Again, the NIR spectral band in Rededge Micasense, used in this version of the 2BDA model in coherence with the formulation initially used for 3BDA, is far away from the optimal 720–740 nm range established by Dall’Olmo and Gitelson [
70] to be used turbid productive waters, but performed better than the version of this algorithm made with the rededge band (2BDA_2). This band is centered in 717 nm, nearer of the optimum range, but the authors describe the aim of this band λ
3 as to be in a spectral region where the absorption by pigments, tripton and CDOM is negligible, which is something that happens at λ > 740 [
21] so the use of longer wavelengths (840 nm in our case) might be feasible. Moreover, as chl-a increases, the λ
3 spectral region of maximal sensitivity to chl-a shifts toward longer wavelengths, and the precise optimal position of λ
1 and λ
3 will depend on the relative importance of the interferences and on the trophic status of the waterbody [
70].
When analyzing the data of both flights in a combined dataset, NIR and red bands got lower correlations than the combination of blue-green and red-green. These last combinations were the best to discriminate the differences between very different chl-a concentrations, being this also reported by Arango and Nairn [
68] with the same sensor. They also developed the models with data of two opposite trophic states and mean chl-a concentrations (oligotrophic: 8.52 µgr/L and eutrophic: 352.60 µgr/L) finding the green and red bands as the most significant.
This finding was used to develop a preliminary ensemble model for the retrieval of chl-a with UAV-Rededege data. In the ensemble model the pixels of the images were first classified in two chl-a classes, and subsequently a second model calibrated for that chl-a class was applied to every pixel to obtain a more accurate result. This model is still to be validated and further research will be done in next years to obtain data in intermediate and higher trophic states chl-a classes.
Our results show that the most suitable algorithms for the three combinations of platforms and sensors were those using NIR and red bands, and that 3BDA and its special case 2BDA were the most successful in retrieving chl-a with all the sensors tested. Beck et al. [
71] obtained similar results when identifying the most “portable” algorithms for the quantification of chl-a. In their study, images from an airborne hyperspectral sensor (CASI) taken simultaneously with dense in situ water quality measurements, and synthetic multispectral datasets, yielded to the conclusion that the NDCI, 2BDA, and 3BDA chl-a algorithms were the most portable, being successful between hyperspectral imagers, WorldView-2 and -3, and Sentinel-2, MERIS-like imagers and, to a lesser degree, MODIS-like imagers.
The overall performance of our approach shows that the use of satellite data as baseline monitoring for eutrophication can be feasible bycombining different sensors, even in very small waterbodies. In this study, we found coherent results when applying the algorithms developed in low to medium chl-a values (1–20 µgr/L) but these failed in retrieving higher concentrations, which could be caused by the “packaging effect” (e.g., [
38,
72]) but also highlights the need for a further development of new algorithms calibrated with higher chl-a data. As it has been stressed in a number of studies, remote sensing reflectance data in waters with a high level of optical complexity are affected by the concentration of different optically active constituents and, as such, a single algorithm is unlikely to perform adequately in all situations [
46,
73]. This issue can be solved by using different algorithms in different conditions, which in our case should be algorithms in high chl-a concentration status, to be used alternatively depending on the limits of applicability stated for each model [
7,
74]. The choice of the appropriate algorithm for each case needs the availability of “a priori data” that could be obtained through the use of autonomous buoys placed in the most representative point of the lake, with real time data acquisition, ingested by the system to apply the suitable algorithm in each case.
The satellite part of our approach has been designed with freely available data, both of global coverage (satellite imagery freely distributed by ESA and NASA) and in situ point measurements (Regional scale. GAIA Portal [
28]). If a compromise between the different agencies responsible for water sampling and analysis could be reached, so that the sampling points were equally representative and valid for use in the calibration and validation (cal/val) of RS products in space and time, and made available as FAIR data, the monitoring of these systems would be enhanced by the spatial component offered by RS through cal/val exercises on a global scale. In this regard, the autonomous buoy networks usually deployed by research institutions, where data are collected at high frequency and made freely available through grassroot networks of global coverage like the Global Lake Observatory Network (GLEON), are of particular interest. These buoys are usually located in central areas of water bodies, i.e., in free-water pixels, perfectly valid for its use as calibration and validation match-up points for RS products.