Hyperspectral Reflectance and Indices for Characterizing the Dynamics of Crop–Weed Competition for Water
Abstract
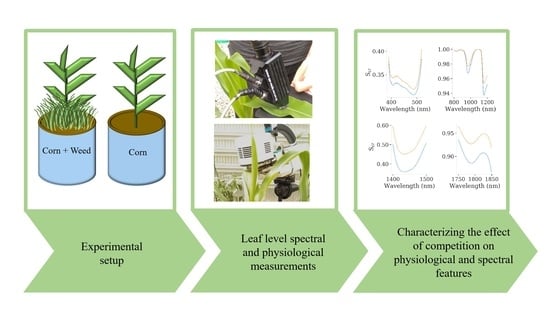
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physiological Measurments
3.2. Hyperspectral Measurements
3.3. Hyperspectral Indices
4. Discussion
4.1. Physiological Properties
4.2. Spectral Characteristics
4.3. Spectral Indices
4.4. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thenkabail, P.S. Optical remote sening of vegetation water content. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2012; pp. 227–244. [Google Scholar]
- Strachan, I.B.; Pattey, E.; Boisvert, J.B. Impact of nitrogen and environmental conditions on corn as detected by hyperspectral reflectance. Remote Sens. Environ. 2002, 80, 213–224. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; John, G.; Lyon, A.H. Detecting Crop Mnagament, Plant Stress, and Disease. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2012; pp. 561–570. [Google Scholar]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, M.; Prasad, Y.G.; Thirupathi, M.; Sreedevi, G.; Dharajothi, B.; Venkateswarlu, B. Use of ground based hyperspectral remote sensing for detection of stress in cotton caused by leafhopper (Hemiptera: Cicadellidae). Comput. Electron. Agric. 2011. [Google Scholar] [CrossRef]
- Neve, P.; Barney, J.N.; Buckley, Y.; Cousens, R.D.; Graham, S.; Jordan, N.R.; Lawton-Rauh, A.; Liebman, M.; Mesgaran, M.B.; Schut, M.; et al. Reviewing research priorities in weed ecology, evolution and management: A horizon scan. Weed Res. 2018, 58, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Behmann, J.; Mahlein, A.K.; Rumpf, T.; Römer, C.; Plümer, L. A review of advanced machine learning methods for the detection of biotic stress in precision crop protection. Precis. Agric. 2015, 16, 239–260. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Wei, X. A review on weed detection using ground-based machine vision and image processing techniques. Comput. Electron. Agric. 2019, 158, 226–240. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, S.Z.; Weise, S.F.; Swanton, C.J. Interference of Redroot Pigweed (Amaranthus retroflexus) in Corn (Zea mays). Weed Sci. 1994. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Weed-Crop Competition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2004; ISBN 0813802792. [Google Scholar]
- Rajcan, I.; Swanton, C.J. Understanding maize-weed competition: Resource competition, light quality and the whole plant. Field Crop. Res. 2001. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, M.; Tattini, M. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Cartfr, G.A.; Paliwal, K.; Pathre, U. Effect of competition and leaf age on visible and infrared reflectance in pine foliage. Plant Cell Environ. 1989, 309–315. [Google Scholar] [CrossRef]
- Liu, W.; Fan, X.; Wang, J.; Zhang, C.; Lu, W.; Gadow, V.; Liu, W.; Fan, X.; Wang, J.; Zhang, C.; et al. Spectral reflectance response of Fraxinus mandshurica leaves to above- and belowground competition. Int. J. Remote Sens. 2012, 1161. [Google Scholar] [CrossRef]
- Leon, C.T.; Shaw, D.R.; Bruce, L.M.; Watson, C. Effect of purple (Cyperus rotundus) and yellow nutsedge (C. esculentus) on growth and reflectance characteristics of cotton and soybean. Weed Sci. 2006. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 2231–2606. [Google Scholar]
- Pietragalla, J.; Mullan, D. Leaf relative water content. In Physiological Breeding II: A Field Guide to Wheat Genotyping; The International Maize and Wheat Improvement Center, CIMMYT: Texcoco, Mexico, 2012. [Google Scholar]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. 1984. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using Near- and Middle-Infrared reflectances. Remote Sens. Environ. 1989, 30. [Google Scholar] [CrossRef]
- Jackson, T.J.; Chen, D.; Cosh, M.; Li, F.; Anderson, M.; Walthall, C.; Doriaswamy, P.; Hunt, E.R. Vegetation water content mapping using Landsat data derived normalized difference water index for corn and soybeans. Remote Sens. Environ. 2004, 92. [Google Scholar] [CrossRef]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. Relations of remote sensing leaf water indices to leaf water thickness in cowpea, bean, and sugarbeet plants. Remote Sens. Environ. 2008, 112. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjärvi, J. Reactive Oxygen Species, Photosynthesis, and Environment in the Regulation of Stomata. Antioxid. Redox Signal. 2019, 30, 1220–1237. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007. [Google Scholar] [CrossRef] [Green Version]
- Thenkabail, P.S.; John, G.; Lyon, A.H. Advances in Hyperspectral Remote Sensing of vegetation and Agricultural Cropland. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2012; pp. 4–31. [Google Scholar]
- Fourty, T.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf Optical Properties with Explicit Description of Its Biochemical Composition: Direct and Inverse Problems. Remote Sens. Environ. 1995, 56, 104–117. [Google Scholar] [CrossRef]
- Buitrago, M.F.; Groen, T.A.; Hecker, C.A.; Skidmore, A.K. Spectroscopic determination of leaf traits using infrared spectra. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 237–250. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Curran, P.J. Remote Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 278, 271–278. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef] [PubMed]






| Measurement | 29 DAS (3–4 Leaves) | 34 DAS (4 Leaves) | 38 DAS (4–5 Leaves) | 43 DAS (4–5 Leaves) | 46 DAS (5–6 Leaves) | 51 DAS (5–6 Leaves) | 54 DAS (5–7 Leaves) | 57 DAS (5–7 Leaves) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w wf | w wf | w wf | w wf | w wf | w wf | w wf | w wf | |||||||||
| Hyperspectral | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 | 9 | 10 | 9 | 10 | - | - |
| Gas exchange | 9 | 10 | - | - | 9 | 9 | 9 | 10 | 9 | 10 | - | - | 9 | 10 | - | - |
| Pigments | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 10 | 10 |
| Relative water content | - | - | - | - | - | - | - | - | 5 | 5 | - | - | - | - | 10 | 10 |
| Biomass | - | - | - | - | - | 10 | 10 | |||||||||
| Index | Formula | Related Variables | Reference |
|---|---|---|---|
| Structure insensitive pigment index (SIPI) | Total pigments | [23] | |
| Plant senescence reflectance index (PSRI) | Total pigments | [24] | |
| Modified anthocyanin reflectance index (mARI) | Anthocyanins | [25] | |
| Carotenoid reflectance index 1 (CRI1) | Carotenoids | [26] | |
| Carotenoid reflectance index 2 (CRI2) | Carotenoids | [26] | |
| Photochemical reflectance index (PRI) | Carotenoids | [27] | |
| Leaf water index (LWI) | Water content | [28] | |
| Moisture stress index (MSI) | Water content | [29] | |
| Normalized difference water index (NDWI) | Water content | [30] | |
| Water band index (WBI) | Water content | [31] |
| Treatment Effect | DAS Effect | Interaction Effect | ||||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Photosynthesis | 61.855 | 0.000 | 29.869 | 0.000 | 5.692 | 0.000 |
| Stomata conductance | 30.553 | 0.000 | 20.131 | 0.000 | 1.561 | 0.192 |
| Intercellular CO2 | 18.996 | 0.000 | 2.859 | 0.028 | 5.974 | 0.000 |
| Treatment Effect | DAS Effect | Interaction Effect | ||||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| SIPI | 0.306 | 0.581 | 6.596 | 0.000 | 0.983 | 0.440 |
| PSRI | 10.371 | 0.002 | 5.276 | 0.000 | 2.259 | 0.042 |
| mARI | 4.031 | 0.047 | 27.072 | 0.000 | 1.813 | 0.102 |
| CRI1 | 1.978 | 0.162 | 15.468 | 0.000 | 0.840 | 0.542 |
| CRI2 | 0.518 | 0.473 | 8.971 | 0.000 | 2.919 | 0.011 |
| PRI | 12.271 | 0.001 | 21.893 | 0.000 | 2.319 | 0.037 |
| LWI | 5.925 | 0.016 | 6.272 | 0.000 | 2.383 | 0.033 |
| MSI | 0.622 | 0.432 | 5.085 | 0.000 | 1.402 | 0.219 |
| NDWI | 0.024 | 0.878 | 6.607 | 0.000 | 1.434 | 0.207 |
| WBI | 0.828 | 0.365 | 4.315 | 0.001 | 1.110 | 0.360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronay, I.; Ephrath, J.E.; Eizenberg, H.; Blumberg, D.G.; Maman, S. Hyperspectral Reflectance and Indices for Characterizing the Dynamics of Crop–Weed Competition for Water. Remote Sens. 2021, 13, 513. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13030513
Ronay I, Ephrath JE, Eizenberg H, Blumberg DG, Maman S. Hyperspectral Reflectance and Indices for Characterizing the Dynamics of Crop–Weed Competition for Water. Remote Sensing. 2021; 13(3):513. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13030513
Chicago/Turabian StyleRonay, Inbal, Jhonathan E. Ephrath, Hanan Eizenberg, Dan G. Blumberg, and Shimrit Maman. 2021. "Hyperspectral Reflectance and Indices for Characterizing the Dynamics of Crop–Weed Competition for Water" Remote Sensing 13, no. 3: 513. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13030513