Dynamics of Vibrio cholerae in a Typical Tropical Lake and Estuarine System: Potential of Remote Sensing for Risk Mapping
Abstract
:1. Introduction
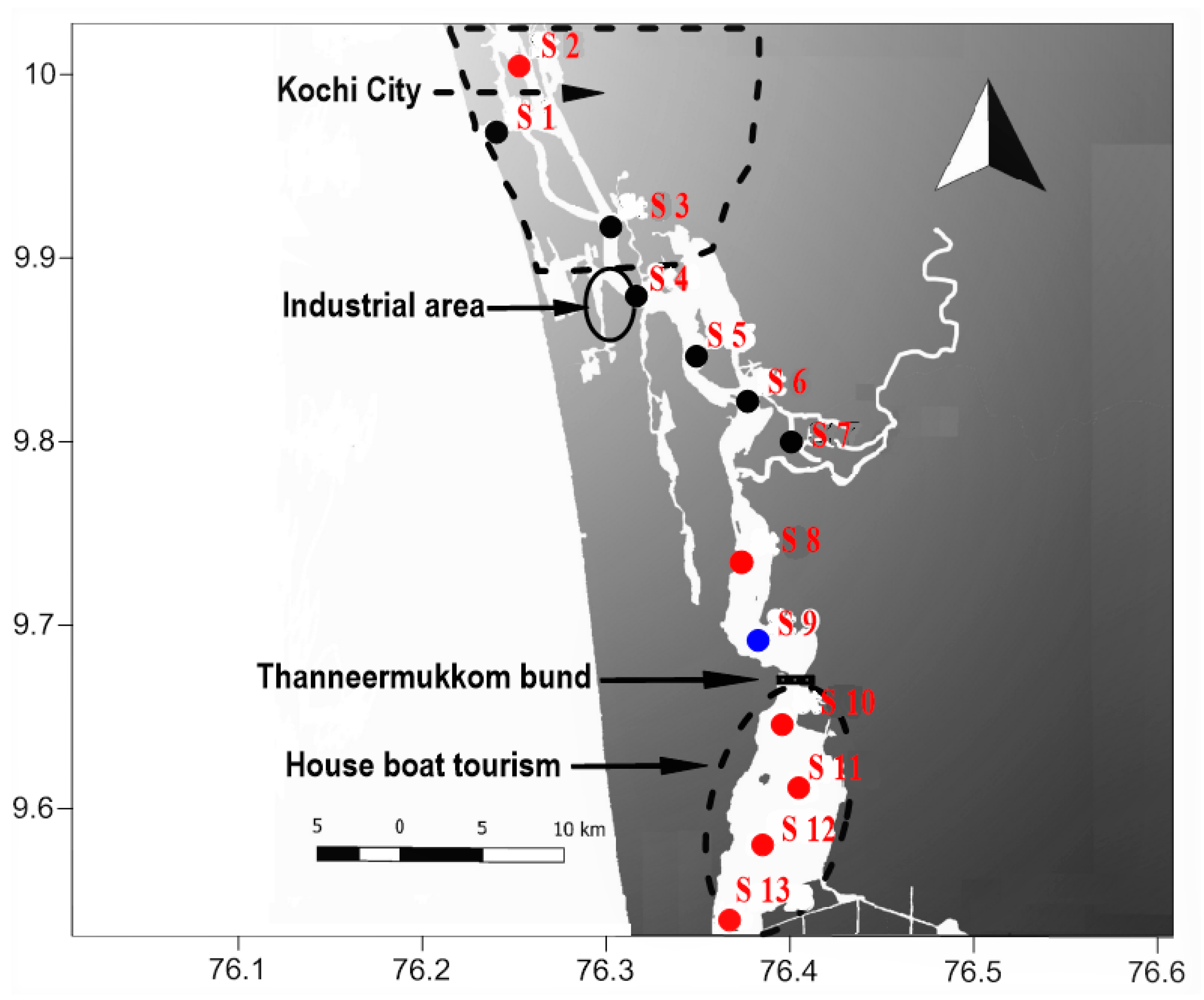
2. Methodology
2.1. Sample Collection and Preservation
2.2. Enumeration of Phytoplankton, Zooplankton and Macrobenthos
2.3. Remote Sensing Observations
2.4. Enumeration of Vibrio cholerae
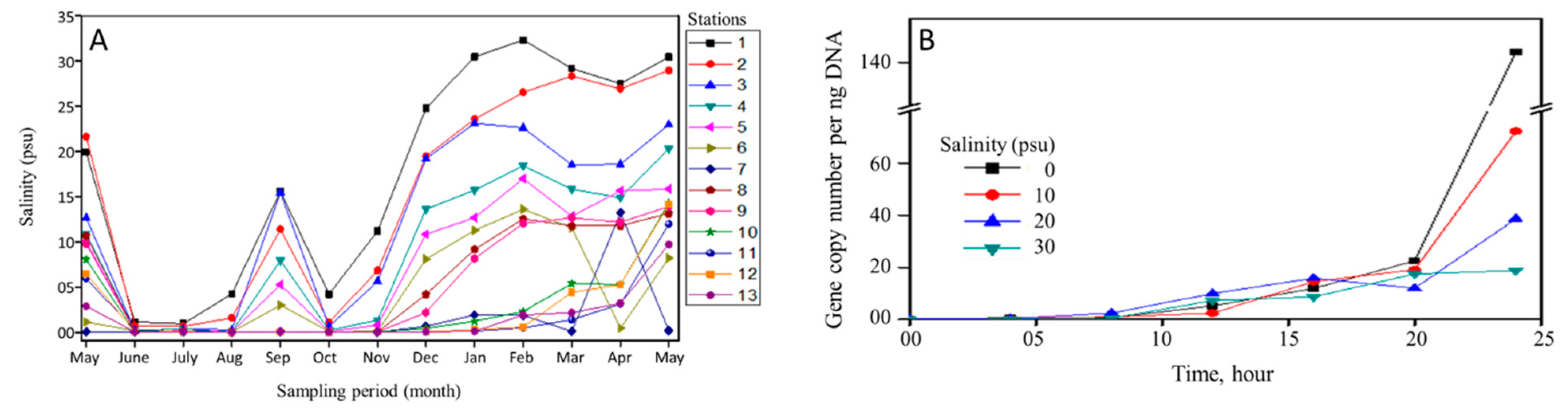
2.5. Salinity Tolerance of V. cholerae
2.6. In Vitro Testing of Chitinase and Laminarinase Activities of V. cholerae
3. Results
3.1. Salinity
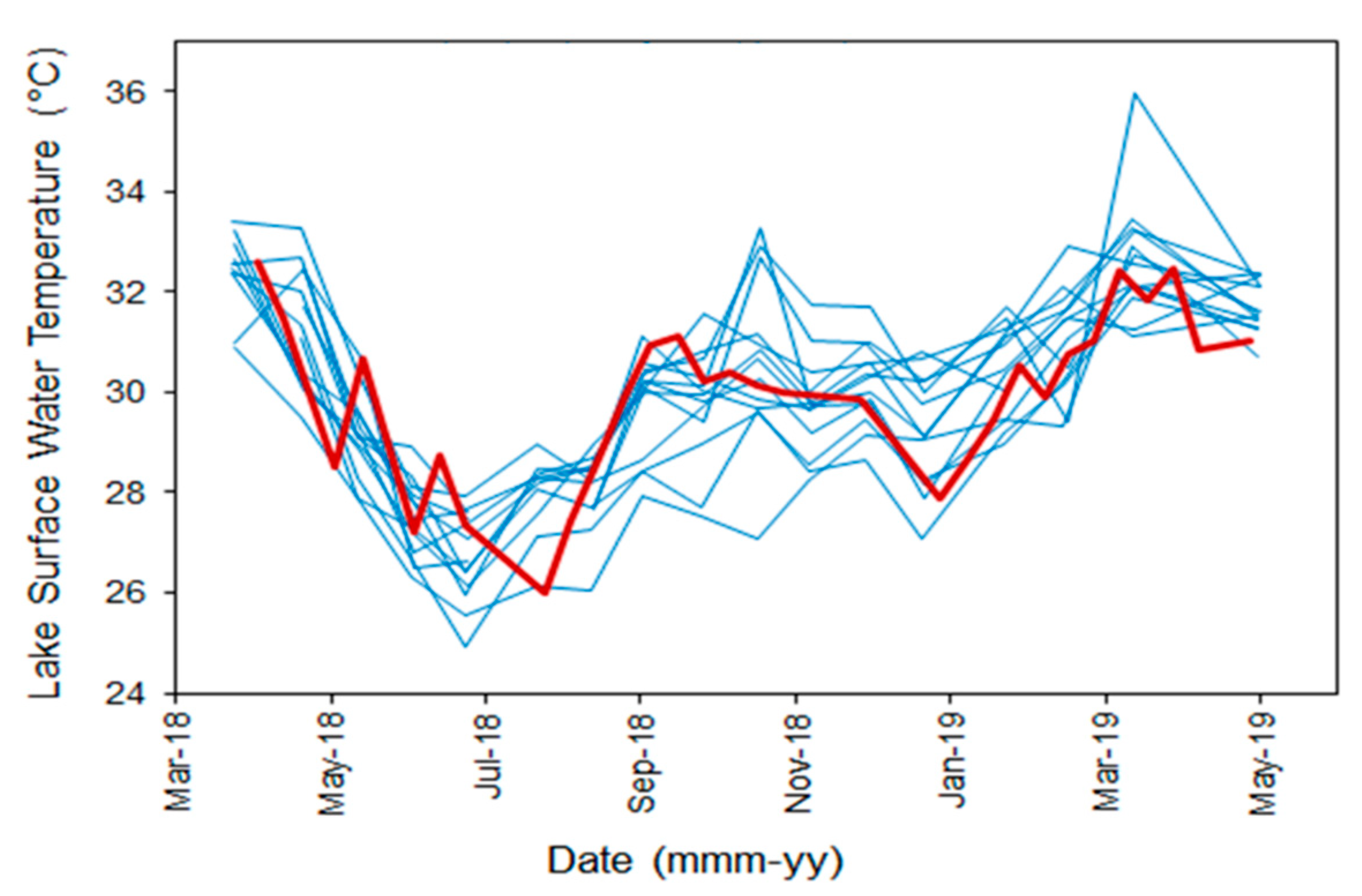
3.2. Surface Water Temperature
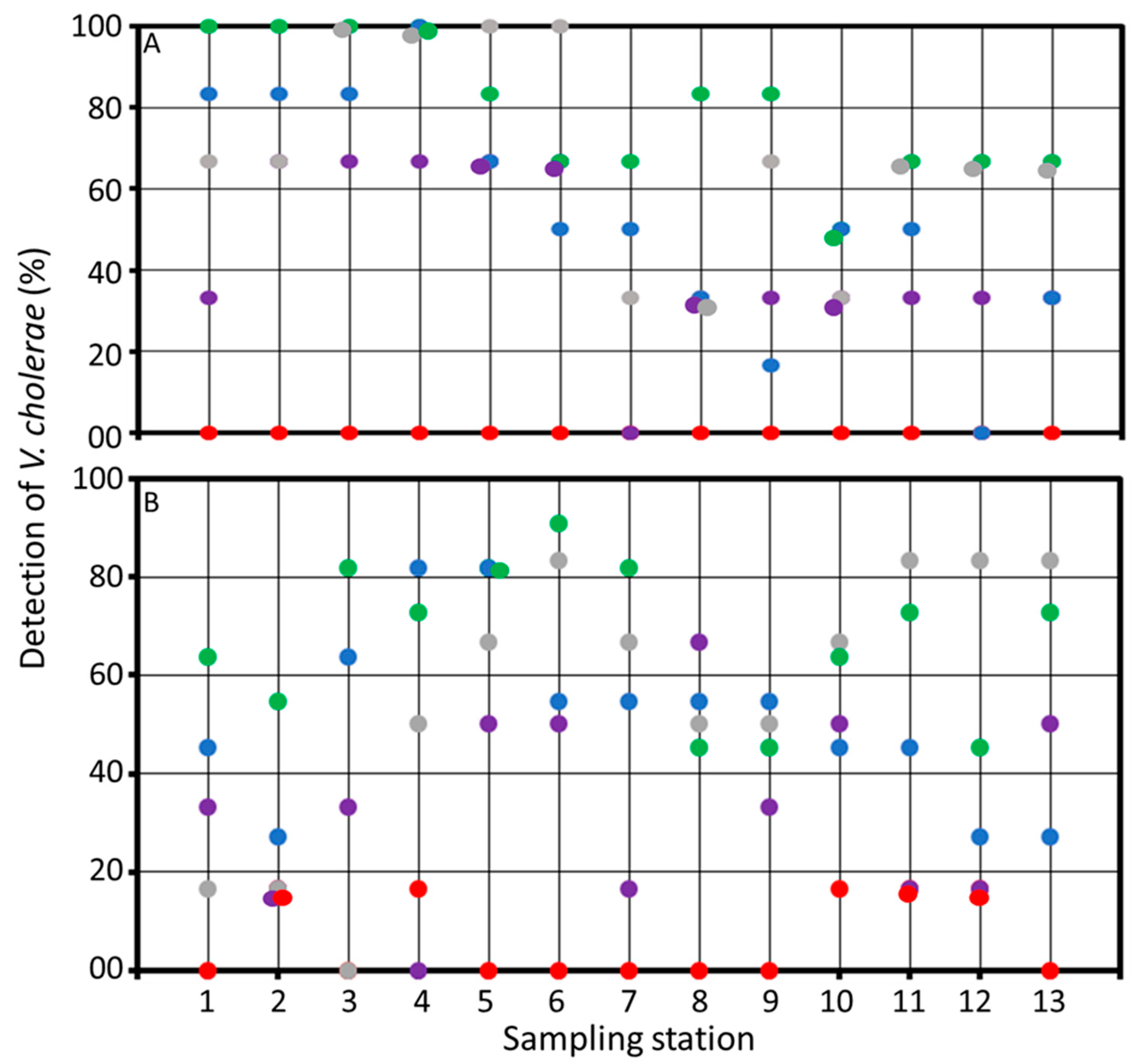
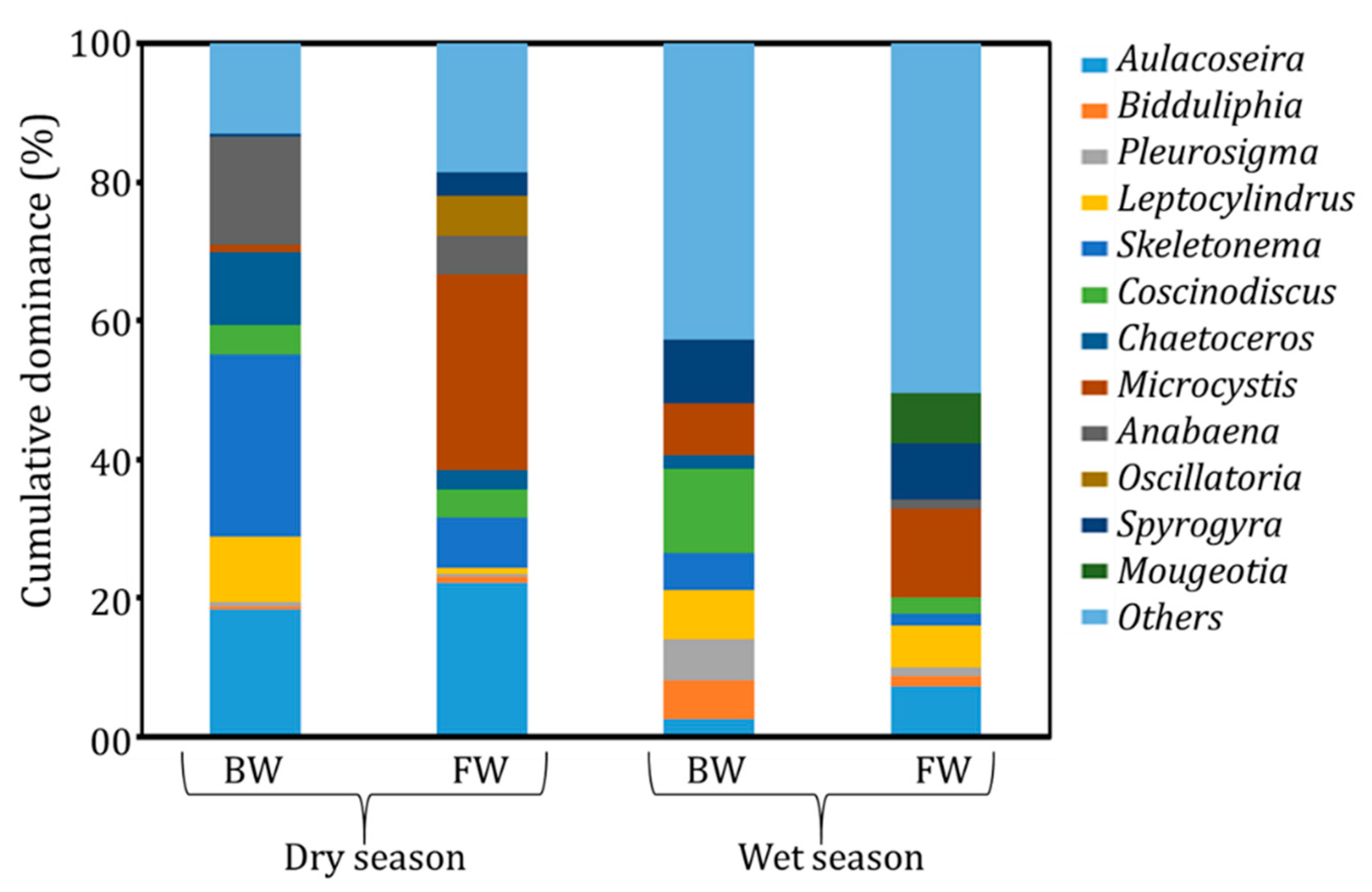
3.3. Environmental Reservoirs of Vibrio cholerae
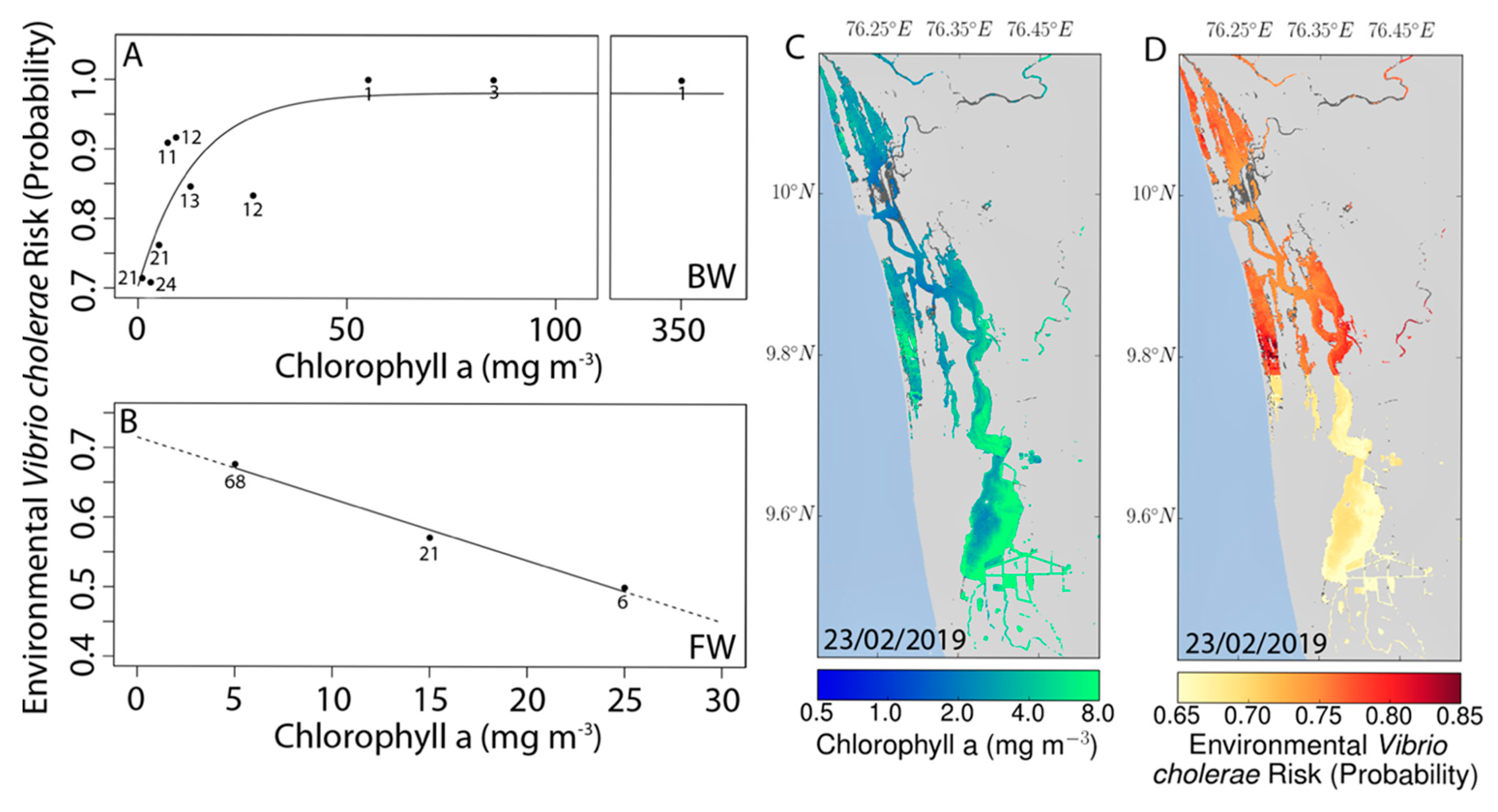
3.4. Remote Sensing and Risk Mapping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Updates Fact Sheet on Cholera (17 January 2019). Available online: https://www.who.int/news-room/fact-sheets/detail/cholera (accessed on 7 March 2021).
- Jutla, A.S.; Akanda, A.S.; Islam, S. Tracking Cholera in Coastal Regions Using Satellite Observations: Tracking Cholera in coastal regions using satellite observations. JAWRA J. Am. Water Resour. Assoc. 2010, 46, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Racault, M.-F.; Abdulaziz, A.; Vijayan, V.; George, G.; Menon, N.; Jasmin, C.; Punathil, M.; McConville, K.; Loveday, B.; Platt, T.; et al. Environmental Reservoirs of Vibrio cholerae: Challenges and Opportunities for Ocean-Color Remote Sensing. Remote Sens. 2019, 11, 2763. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- GTFCC/WHO. Ending Cholera: A Global Road Map to 2030; WHO: Geneva, Switzerland, 2017; 32p. [Google Scholar]
- Islam, M.S.; Alam, M.J.; Khan, S.I. Occurence and distribution of culturable Vibrio cholerae O1 in aquatic environments of Bangladesh. Int. J. Environ. Stud. 1995, 47, 217–223. [Google Scholar] [CrossRef]
- Jutla, A.; Khan, R.; Colwell, R. Natural Disasters and Cholera Outbreaks: Current Understanding and Future Outlook. Curr. Environ. Health Rep. 2017, 4, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lobitz, B.; Beck, L.; Huq, A.; Wood, B.; Fuchs, G.; Faruque, A.S.G.; Colwell, R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 2000, 97, 1438–1443. [Google Scholar] [CrossRef] [Green Version]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean Warming and Spread of Pathogenic Vibrios in the Aquatic Environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Sathyendranath, S.; Abdulaziz, A.; Menon, N.; George, G.; Evers-King, H.; Kulk, G.; Colwell, R.; Jutla, A.; Platt, T. Building Capacity and Resilience Against Diseases Transmitted via Water Under Climate Perturbations and Extreme Weather Stress. In Space Capacity Building in the XXI Century; Ferretti, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 281–298. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Sharma, N.C. Cholerae Outbreaks in India; Balakrish Nair, G., Takeda, Y., Eds.; Springer: New York, NY, USA, 2014; Volume 379, 259p. [Google Scholar]
- Reed, R.H.; Singh, I.S.B.; Mani, S.K. Solar Disinfection of Drinking Water: Lessons from Field Studies in India. In Progress on Drinking Water Research; Lefebvre, M.H., Roux, M.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2008; Volume 1, 290p. [Google Scholar]
- Gireesh, R.; Varghese, M.; Thomas, V.J. Phytoplankton—Collection, Estimation, Classification and Diversity. In Proceedings of the Summer School on Recent Advances in Marine Biodiversity Conservation and Management, Kochi, India, 16 February–8 March 2015; Central Marine Fisheries Research Institute: Kochi, India, 2015; pp. 24–28. [Google Scholar]
- Gopinathan, C.P. A systematic account of the littoral diatoms of the southwest coast of India. J. Mar. Biol. Assoc. India 1984, 26, 1–31. [Google Scholar]
- Thomas, R. Identifying Marine Phytoplankton; Academic Press: California, CA, USA, 1997. [Google Scholar]
- Throndsen, J.; Hasle, G.R.; Tangen, K. Phytoplankton of Norwegian Coastal Waters; Almater Forlag AS: Oslo, Norway, 2007. [Google Scholar]
- Parson, T.R.; Maita, Y.; Lalli, C.M. A Manual of Biological and Chemical Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; 173p. [Google Scholar]
- George, G.; Sreeraj, C.R.; Dam Roy, S. Brachionid rotifer diversity in Andaman waters. Indian J. Geo. Mar. Sci. 2011, 40, 454–459. [Google Scholar]
- Varghese, M.; Thomas, V.J.; Susan, V. Collection and Estimation of Zooplankton. In Proceedings of the Summer School on Recent Advances in Marine Biodiversity Conservation and Management, Cochin, India, 16 February–8 March 2015; Central Marine Fisheries Research Institute: Kochi, India, 2015; pp. 29–32. [Google Scholar]
- Carrea, L.; Embury, O.; Merchant, C.J. Datasets related to in-land water for limnology and remote sensing applications: Distance-to-land, distance-to-water, water-body identifier and lake-centre co-ordinates. Geosci. Data J. 2015, 2, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Maccallum, S.N.; Merchant, C.J. Surface water temperature observations of large lakes by optimal estimation. Can. J. Remote Sens. 2012, 38, 25–45. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. ACOLITE for Sentinel-2: Aquatic applications of MSI imagery. In Proceedings of the 2016-ESA Living Planet Symposium, Prague, Czech Republic, 9–13 May 2016; p. SP740. [Google Scholar]
- Vanhellemont, Q.; Ruddick, K. Atmospheric correction of metre-scale optical satellite data for inland and coastal water applications. Remote Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Siegel, D.A.; O’Brien, M.C.; Toole, D.; Greg Mitchell, B.; Kahru, M.; Chavez, F.P.; Strutton, P.; Cota, G.F.; et al. (Eds.) SeaWiFS Postlaunch Calibration and Validation Analyses; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2000; Volume 11, 49p.
- Franz, B.A.; Bailey, S.W.; Kuring, N.; Werdell, P.J. Ocean colour measurements with the operational land imager on Landsat-8: Implementation and evaluation in SeaDAS. J. Appl. Remote Sens. 2015, 9, 096070. [Google Scholar] [CrossRef]
- Peng, X.; Yu, K.-Q.; Deng, G.-H.; Jiang, Y.-X.; Wang, Y.; Zhang, G.-X.; Zhou, H.-W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods 2013, 95, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, J.F.; Morsali, P.; Nejad, H.R.; Fooladi, A.A.I.; Najafy, S. Evaluation of new primers for detecting toxigenic vibrio cholerae by multiplex PCR. Microbiol. Res. 2011, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Ang, G.Y.; Yu, C.Y.; Balqis, K.; Elina, H.T.; Azura, H.; Hani, M.H.; Yean, C.Y. Molecular Evidence of Cholera Outbreak Caused by a Toxigenic Vibrio cholerae O1 El Tor Variant Strain in Kelantan, Malaysia. J. Clin. Microbiol. 2010, 48, 3963–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, D.M. Exocellular Chitinase from a Streptomyces sp. J. Gen. Microbiol. 1954, 11, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Ramamurthy, T.; Mutreja, A.; Weill, F.-X.; Das, B.; Ghosh, A.; Nair, G.B. Revisiting the Global Epidemiology of Cholera in Conjuction With the Genomics of Vibrio cholerae. Front. Public Health 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Ryan, S.J.; Stewart-Ibarra, A.M.; Finkelstein, J.L.; King, C.A.; Qiao, H.; Polhemus, M.E. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 2015, 149, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, P.; Sarma, K.; George, G.; Krishnan, P.; Roy, S.D.; Sankar, K. Impact of Coastal Pollution on Microbial and Mineral Profile of Edible Oyster (Crassostrea rivularis) in the Coastal Waters of Andaman. Bull. Environ. Contam. Toxicol. 2015, 95, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WISA. Vembanad-Kol Wetlands—An Integrated Management Planning Framework for Conservation and Wise Use; Wetland International-South AISA: New Delhi, India, 2013; 113p. [Google Scholar]
- Louis, V.R.; Russek-Cohen, E.; Choopun, N.; Rivera, I.N.G.; Gangle, B.; Jiang, S.C.; Rubin, A.; Patz, J.A.; Huq, A.; Colwell, R.R. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 2003, 69, 2773–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, F.L.; Attwell, R.; Jangi, S.; Colwell, R.R. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 1982, 44, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huq, A.; West, P.A.; Small, E.B.; Huq, M.I.; Colwell, R.R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl. Environ. Microbiol. 1984, 48, 420–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materna, A.C.; Friedman, J.; Bauer, C.; David, C.; Chen, S.; Huang, I.B.; Gillens, A.; Clarke, S.A.; Polz, M.F.; Alm, E.J. Shape and evolution of the fundamental niche in marine Vibrio. ISME J. 2012, 6, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, G.E. Concluding Remarks in Cold Spring Harbour Syposium on Quantitative Biology. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Höfle, M.G.; Pruzzo, C. Long-term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME J. 2011, 6, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Levy, S. Warming Trend: How Climate ShapesVibrioEcology. Environ. Health Perspect. 2015, 123, A82–A89. [Google Scholar] [CrossRef] [Green Version]
- Correspondent, S. Proximity of Wells to Septic Tanks in Flood-Hit Areas Raises Concern. The Hindu, 7 September 2018. [Google Scholar]
- Kanungo, S.; Sah, B.K.; Lopez, A.L.; Sung, J.S.; Paisley, A.M.; Sur, D.; Clemens, J.D.; Nair, G.B. Cholera in India: An analysis of reports, 1997–2006. Bull. World Health Organ. 2010, 88, 185–191. [Google Scholar] [CrossRef]
- Finkeletein, R.A. Cholera, Vibrio Cholera O1 and O139, and Other Pathogenic Vibrios. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Huston, TX, USA, 1996. [Google Scholar]
- Pruzzo, C.; Vezzulli, L.; Colwell, R.R. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 2008, 10, 1400–1410. [Google Scholar] [CrossRef]
- Vezzulli, L.; Pruzzo, C.; Huq, A.; Colwell, R.R. Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ. Microbiol. Rep. 2010, 2, 27–33. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Van Rijssel, M.; Bolhuis, H. Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol. Ecol. 2007, 59, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lin, H.; Wang, X.; Austin, B. Significance of Vibrio species in the marine organic carbon cycle—A review. Sci. China Earth Sci. 2018, 61, 1357–1368. [Google Scholar] [CrossRef]
- Turner, J.W.; Malayil, L.; Guadagnoli, D.; Cole, D.; Lipp, E.K. Detection of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae with respect to seasonal fluctuations in temperature and plankton abundance. Environ. Microbiol. 2014, 16, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Finger, F.; Knox, A.; Bertuzzo, E.; Mari, L.; Bompangue, D.; Gatto, M.; Rodriguez-Iturbe, I.; Rinaldo, A. Cholera in the Lake Kivu region (DRC): Integrating remote sensing and spatially explicit epidemiological modeling. Water Resour. Res. 2014, 50, 5624–5637. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Cao, C.X.; Wang, D.C.; Kan, B.; Xu, Y.F.; Ni, X.L.; Zhu, Z.C. Environmental factor analysis of cholera in China using remote sensing and geographical information systems. Epidemiol. Infect. 2016, 144, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyendranath, S. Remote Sensing of Ocean Colour in Coastal, and Other Optically Complex, Waters; IOCCCG: Dartmouth, NS, Canada, 2000; Volume 3. [Google Scholar]
- Haldar, R.; Khosa, R.; Gosain, A.K. Impact of Anthropogenic Interventions on the Vembanad Lake System. In Water Resources and Environmental Engineering I: Surface and Groundwater; Rathinasamy, M., Chandramouli, S., Phanindra, K., Mahesh, U., Eds.; Springer: Singapore, 2019; Volume I, pp. 9–99. [Google Scholar]
- Durkin, C.A.; Mock, T.; Armbrust, E.V. Chitin in Diatoms and Its Association with the Cell Wall. Eukaryot. Cell 2009, 8, 1038–1050. [Google Scholar] [CrossRef] [Green Version]
- Neogi, S.B.; Islam, M.S.; Nair, G.B.; Yamasaki, S.; Lara, R.J. Occurrence and distribution of plankton-associated and free-living toxigenic Vibrio cholerae in a tropical estuary of a cholera endemic zone. Wetl. Ecol. Manag. 2012, 20, 271–285. [Google Scholar] [CrossRef]
- Turner, J.W.; Good, B.; Cole, D.; Lipp, E.K. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J. 2009, 3, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, M.; Asplund, M.E.; Karunasagar, I.; Rehnstam-Holm, A.-S.; Godhe, A. Prorocentrum micans promote and Skeletonema tropicum disfavours persistence of the pathogenic bacteria Vibrio parahaemolyticus. Indian J. Geo. Mar. Sci. 2013, 42, 729–733. [Google Scholar]
- Wang, S.; Said, I.H.; Thorstenson, C.; Thomsen, C.; Ullrich, M.S.; Kuhnert, N.; Thomsen, L. Pilot-scale production of antibacterial substances by the marine diatom Phaeodactylum tricornutum Bohlin. Algal Res. 2018, 32, 113–120. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Agrawal, M.K.; Bagchi, S.N. Microcystin-producing and non-producing cyanobacterial blooms collected from the Central India harbor potentially pathogenic Vibrio cholerae. Ecotoxicol. Environ. Saf. 2015, 115, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Goldar, M.M.; Morshed, M.G.; Bakht, H.B.M.; Sack, D.A. Chemotaxis between Vibrio cholerae O1 and a blue-green alga, Anabaena sp. Epidemiol. Infect. 2006, 134, 645–648. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, M.; Ahmed, N.; Clemens, J. Environmental reservoirs of Vibrio cholerae. Vaccine 2019, 38, A52–A62. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Drasar, B.S.; Bradley, D.J. Long-term persistence of toxigenic Vibrio cholerae O1 in the mucilagenous sheeth of a blue-green alga, Anabaena variabilis. J. Trop. Med. Hyg. 1990, 93, 133–139. [Google Scholar] [PubMed]
- Islam, M.S.; Goldar, M.M.; Morshed, M.G.; Khan, M.N.; Islam, M.S.; Sack, R. Involvement of the hap gene (mucinase) in the survival of Vibrio cholerae O1 in association with the blue-green alga, Anabaena sp. Can. J. Microbiol. 2002, 48, 793–800. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anas, A.; Krishna, K.; Vijayakumar, S.; George, G.; Menon, N.; Kulk, G.; Chekidhenkuzhiyil, J.; Ciambelli, A.; Kuttiyilmemuriyil Vikraman, H.; Tharakan, B.; et al. Dynamics of Vibrio cholerae in a Typical Tropical Lake and Estuarine System: Potential of Remote Sensing for Risk Mapping. Remote Sens. 2021, 13, 1034. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13051034
Anas A, Krishna K, Vijayakumar S, George G, Menon N, Kulk G, Chekidhenkuzhiyil J, Ciambelli A, Kuttiyilmemuriyil Vikraman H, Tharakan B, et al. Dynamics of Vibrio cholerae in a Typical Tropical Lake and Estuarine System: Potential of Remote Sensing for Risk Mapping. Remote Sensing. 2021; 13(5):1034. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13051034
Chicago/Turabian StyleAnas, Abdulaziz, Kiran Krishna, Syamkumar Vijayakumar, Grinson George, Nandini Menon, Gemma Kulk, Jasmin Chekidhenkuzhiyil, Angelo Ciambelli, Hridya Kuttiyilmemuriyil Vikraman, Balu Tharakan, and et al. 2021. "Dynamics of Vibrio cholerae in a Typical Tropical Lake and Estuarine System: Potential of Remote Sensing for Risk Mapping" Remote Sensing 13, no. 5: 1034. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13051034








