Identifying Algal Bloom ‘Hotspots’ in Marginal Productive Seas: A Review and Geospatial Analysis
Abstract
:1. Introduction
2. Background: Northern Indian Ocean (NIO)
3. Data and Methods
- Search strategy and data extraction
- Mapping the frequency of algal blooms
- Satellite data acquisition
- Samples Preparation for SEM images of species
4. Exuberant Growth of Algae
4.1. Nutrients
4.2. Sunlight
- (1)
- (2)
- By depositing iron, phytoplankton growth is increased, leading to the formation of Dimethylsulfoniopropionate (DMSP) that, when degraded to dimethyl sulphide (DMS), can lead to acidic sulphate aerosols [69]. Aerosols can reduce the shortwave radiation arriving at the surface by 21 W m−2 and increase top-of-the-atmosphere-reflected radiation by 18 W m−2 during March–April [70];
- (3)
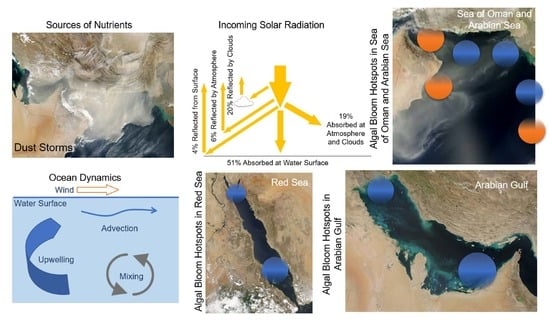
- Anthropogenic aerosols have caused a 20 W m−2 (10%) fall in solar radiation over the Arabian Sea during the period 1950 to 2000, due to the 3 km thick layer of pollution over the northern Indian Ocean (south India) [71,72]. This must certainly affect ocean productivity and the types of marine organisms that are found in the NIO. For example, during SWMs, solar radiation is prominent in the Red Sea (hotspots 9 and 10), Arabian Gulf (hotspot 7), and Sea of Oman (hotspot 6). However, during NEMs, solar radiation is prominent in the Arabian Sea (hotspot 5).
5. Transport of Algae and Nutrients
5.1. Upwelling
5.2. Convective Mixing
5.3. Advection
6. Hotspots of Algal Blooms in NIO
6.1. Southwest Monsoon
6.2. Northwest Monsoon
7. Biological Makeup of Algal Bloom Genera
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Madhupratap, M.; Sawant, S.; Gauns, M. A First Report on a Bloom of the Marine Prymnesiophycean, Phaeocystis Globosa from the Arabian Sea. Oceanol. Acta 2000, 23, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Al-Azri, A.; Al-Hashmi, K.; Goes, J.; Gomes, H.; Rushdi, A.I.; Al-Habsi, H.; Al-Khusaibi, S.; Al-Kindi, R.; Al-Azri, N. Seasonality of the Bloom-Forming Heterotrophic Dinoflagellate Noctiluca Scintillans in the Gulf of Oman in Relation to Environmental Conditions. Int. J. Ocean. Oceanogr. 2007, 2, 51–60. [Google Scholar]
- Rao, S.; Al-Yamani, F. Phytoplankton Ecology in the Waters between Shatt Al-Arab and Straits of Hormuz, Arabian Gulf: A Review. Plankton Biol. Ecol. 1998, 45, 101–116. [Google Scholar]
- Luqman, M.; Javed, M.M.; Yousafzai, A.; Saeed, M.; Ahmad, J.; Chaghtai, F. Blooms of Pollution Indicator Micro-Alga (Synedra acus) in Northern Arabian Sea along Karachi, Pakistan. Indian J. Geo-Mar. Sci. 2015, 44, 1377–1381. [Google Scholar]
- Jin, Q.; Wang, C. A Revival of Indian Summer Monsoon Rainfall since 2002. Nat. Clim. Chang. 2017, 7, 587. [Google Scholar] [CrossRef]
- Wang, H.; McClean, J.L.; Talley, L.D.; Yeager, S. Seasonal Cycle and Annual Reversal of the Somali Current in an Eddy-Resolving Global Ocean Model. J. Geophys. Res. Ocean. 2018, 123, 6562–6580. [Google Scholar] [CrossRef]
- Li, Z.; Lau, W.; Ramanathan, V.; Wu, G.; Ding, Y.; Manoj, M.G.; Liu, J.; Qian, Y.; Li, J.; Zhou, T. Aerosol and Monsoon Climate Interactions over Asia. Rev. Geophys. 2016, 54, 866–929. [Google Scholar] [CrossRef]
- Singh, A.; Ramesh, R. Environmental Controls on New and Primary Production in the Northern Indian Ocean. Prog. Oceanogr. 2015, 131, 138–145. [Google Scholar] [CrossRef]
- Sarma, V.V.S.S.; Swathi, P.S.; Kumar, M.D.; Prasannakumar, S.; Bhattathiri, P.M.A.; Madhupratap, M.; Ramaswamy, V.; Sarin, M.M.; Gauns, M.; Ramaiah, N.; et al. Carbon Budget in the Eastern and Central Arabian Sea: An Indian JGOFS Synthesis. Glob. Biogeochem. Cycles 2003, 17, 1102. [Google Scholar] [CrossRef]
- Zhai, P.; Bower, A.S.; Smethie, W.M.; Pratt, L.J. Formation and Spreading of Red Sea Outflow Water in the Red Sea. J. Geophys. Res. Ocean. 2015, 120, 6542–6563. [Google Scholar] [CrossRef]
- Alosairi, Y.; Imberger, J.; Falconer, R.A. Mixing and Flushing in the Persian Gulf (Arabian Gulf). J. Geophys. Res. Ocean. 2011, 116, C03029. [Google Scholar] [CrossRef]
- Bruce, J.G.; Johnson, D.R.; Kindle, J.C. Evidence for Eddy Formation in the Eastern Arabian Sea during the Northeast Monsoon. J. Geophys. Res. 1994, 99, 7651–7664. [Google Scholar] [CrossRef]
- Kumar, S.P.; Ramaiah, N.; Gauns, M.; Sarma, V.V.S.S.; Muraleedharan, P.M.; Raghukumar, S.; Kumar, M.D.; Madhupratap, M. Physical Forcing of Biological Productivity in the Northern Arabian Sea during the Northeast Monsoon. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 1115–1126. [Google Scholar] [CrossRef]
- Zaman, Q.; Afzal, M.; Haroon, M.A. Characteristics of Sea Surface Temperature of the Arabian Sea Coast of Pakistan and Impact of Tropical Cyclones on SST. Pak. J. Meteorol. 2014, 11, 61–70. [Google Scholar]
- Nandkeolyar, N.; Raman, M.; Sandhya Kiran, G.; Ajai, A. Comparative Analysis of Sea Surface Temperature Pattern in the Eastern and Western Gulfs of Arabian Sea and the Red Sea in Recent Past Using Satellite Data. Int. J. Oceanogr. 2013, 2013, 16. [Google Scholar] [CrossRef]
- Godad, S.P.; Panmei, C.; Naidu, P.D. Remote Forcing of Winter Cooling in the Arabian Sea: Implications for the NE Monsoon. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 586, 110755. [Google Scholar] [CrossRef]
- Murty, S.A.; Bernstein, W.N.; Ossolinski, J.E.; Davis, R.S.; Goodkin, N.F.; Hughen, K.A. Spatial and Temporal Robustness of Sr/Ca-SST Calibrations in Red Sea Corals: Evidence for Influence of Mean Annual Temperature on Calibration Slopes. Paleoceanogr. Paleoclimatol. 2018, 33, 443–456. [Google Scholar] [CrossRef]
- Forouzan, F.; Ghazban, F.; Ardestani, M. Sea Surface Temperature and Salinity Reconstruction from Geochemical Tracers of a Massive Coral in the Persian Gulf. Int. J. Environ. Res. 2014, 8, 1135–1144. [Google Scholar]
- Al Shehhi, M.R.; Gherboudj, I.; Ghedira, H. In Situ Spectral Response of the Arabian Gulf and Sea of Oman Coastal Waters to Bio-Optical Properties. J. Photochem. Photobiol. B Biol. 2017, 175, 235–243. [Google Scholar] [CrossRef]
- Rao, G.R.; Devi, Y.V.; Reddy, K.G.; Prasad, A.L.N. Studies on Evaporation from the North Indian Ocean. Indian J. Mar. Sci. 1981, 10, 337–340. [Google Scholar]
- Xue, P.; Eltahir, E.A.B. Estimation of the Heat and Water Budgets of the Persian (Arabian) Gulf Using a Regional Climate Model. J. Clim. 2015, 28, 5041–5062. [Google Scholar] [CrossRef]
- Al-Subhi, A.M. Estimation of Evaporation Rates in the Southern Red Sea Based on the AVHRR Sea Surface Temperature Data. J. King Abdulaziz Univ. Mar. Sci. 2012, 23, 77–89. [Google Scholar] [CrossRef]
- Zhai, P. Buoyancy-Driven Circulation in the Red Sea. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2014. [Google Scholar]
- Wiggert, J.D.; Hood, R.R.; Banse, K.; Kindle, J.C. Monsoon-Driven Biogeochemical Processes in the Arabian Sea. Prog. Oceanogr. 2005, 65, 176–213. [Google Scholar] [CrossRef]
- McCreary, J.P.; Kohler, K.E.; Hood, R.R.; Olson, D.B. A Four-Component Ecosystem Model of Biological Activity in the Arabian Sea. Prog. Oceanogr. 1996, 37, 193–240. [Google Scholar] [CrossRef]
- Volpe, G.; Colella, S.; Forneris, V.; Tronconi, C.; Santoleri, R. The Mediterranean Ocean Colour Observing System–System Development and Product Validation. Ocean Sci. 2012, 8, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Joseph, T.; Shaiju, P.; Laluraj, C.M.; Balachandran, K.K.; Nair, M.; George, R.; Nair, K.K.C.; Sahayak, S.; Prabhakaran, M.P. Nutrient Environment of Red Tide-Infested Waters off South-West Coast of India. Environ. Monit. Assess. 2008, 143, 355–361. [Google Scholar] [CrossRef]
- Brock, J.C.; McClain, C.R.; Luther, M.E.; Hay, W.W. The Phytoplankton Bloom in the Northwestern Arabian Sea during the Southwest Monsoon of 1979. J. Geophys. Res. Ocean. 1991, 96, 20623–20642. [Google Scholar] [CrossRef]
- Shriadah, M.A. Nutrient Salts in the United Arab Emirates Waters (the Arabian Gulf and the Gulf of Oman). Pak. J. Mar. Sci. 2006, 15, 1–9. [Google Scholar]
- Shaikh, E.A.; Roff, J.C.; Dowidar, N.M. Phytoplankton Ecology and Production in the Red Sea off Jiddah, Saudi Arabia. Mar. Biol. 1986, 92, 405–416. [Google Scholar] [CrossRef]
- Khalid, S.; Qasim, M.; Farhan, D. Hydro-Meteorological Characteristics of Indus River Basin at Extreme North of Pakistan. J. Earth Sci. Clim. Chang. 2013, 5, 170. [Google Scholar]
- Al-Handal, A.; Hu, C. MODIS Observations of Human-Induced Changes in the Mesopotamian Marshes in Iraq. Wetlands 2015, 35, 31–40. [Google Scholar] [CrossRef]
- Harrison, P.J.; Khan, N.; Yin, K.; Saleem, M.; Bano, N.; Nisa, M.; Ahmed, S.I.; Rizvi, N.; Azam, F. Nutrient and Phytoplankton Dynamics in Two Mangrove Tidal Creeks of the Indus River Delta, Pakistan. Mar. Ecol. Prog. Ser. 1997, 157, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Al-Shehhi, M.R.; Gherboudj, I.; Ghedira, H. Temporal-Spatial Analysis of Chlorophyll Concentration Associated with Dust and Wind Characteristics in the Arabian Gulf. In Proceedings of the 2012 Oceans-Yeosu IEEE, Yeosu, Korea, 21–24 May 2012; pp. 1–6. [Google Scholar]
- Patra, P.K.; Behera, S.K.; Herman, J.R.; Maksyutov, S.; Akimoto, H.; Yamagata, Y. The Indian Summer Monsoon Rainfall: Interplay of Coupled Dynamics, Radiation and Cloud Microphysics. Atmos. Chem. Phys. 2005, 5, 2181–2188. [Google Scholar] [CrossRef] [Green Version]
- Kalenderski, S.; Stenchikov, G.L.; Zhao, C. Modeling a Typical Winter-Time Dust Event over the Arabian Peninsula and the Red Sea. Atmos. Chem. Phys. 2013, 13, 1999–2014. [Google Scholar] [CrossRef] [Green Version]
- Wiggert, J.D.; Murtugudde, R.G. The Sensitivity of the Southwest Monsoon Phytoplankton Bloom to Variations in Aeolian Iron Deposition over the Arabian Sea. J. Geophys. Res. Ocean. 2007, 112, C05005. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.V.S.; Al-Yamani, F.; Rao, C.V.N. Eolian Dust Affects Phytoplankton in the Waters off Kuwait, the Arabian Gulf. Naturwissenschaften 1999, 86, 525–529. [Google Scholar]
- Kumar, A. Reclaimed Islands and New Offshore Townships in the Arabian Gulf: Potential Natural Hazards. Curr. Sci. 2009, 96, 480. [Google Scholar]
- Anisimov, A.; Tao, W.; Stenchikov, G.; Kalenderski, S.; Prakash, P.J.; Yang, Z.-L.; Shi, M. Quantifying Local-Scale Dust Emission from the Arabian Red Sea Coastal Plain. Atmos. Chem. Phys. 2017, 17, 993–1015. [Google Scholar] [CrossRef] [Green Version]
- Al-Shehhi, M.R.; Gherboudj, I.; Ghedira, H. A Study on the Effect of Dust and Wind on Phytoplankton Activities in the Arabian Gulf. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 2571–2574. [Google Scholar]
- Sahay, A.; Ali, S.M.; Gupta, A.; Goes, J.I. Ocean Color Satellite Determinations of Phytoplankton Size Class in the Arabian Sea during the Winter Monsoon. Remote Sens. Environ. 2017, 198, 286–296. [Google Scholar] [CrossRef]
- Sale, P.F.; Feary, D.A.; Burt, J.A.; Bauman, A.G.; Cavalcante, G.H.; Drouillard, K.G.; Kjerfve, B.; Marquis, E.; Trick, C.G.; Usseglio, P. The Growing Need for Sustainable Ecological Management of Marine Communities of the Persian Gulf. Ambio 2011, 40, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Heil, C.A.; Glibert, P.M.; Al-Sarawl, M.A.; Faraj, M.; Behbehani, M.; Husain, M. First Record of a Fish-Killing Gymnodinium sp. Bloom in Kuwait Bay, Arabian Sea: Chronology and Potential Causes. Mar. Ecol. Ser. 2001, 214, 15–23. [Google Scholar] [CrossRef]
- Mansour, A.M.; Nawar, A.H.; Madkour, H.A. Metal Pollution in Marine Sediments of Selected Harbours and Industrial Areas along the Red Sea Coast of Egypt. Ann. Nat. Mus. Wien Ser. A Mineral. Petrogr. Geol. Paläontol. Anthropol. Prähist. 2011, 113, 225–244. [Google Scholar]
- Naser, H. Marine Ecosystem Diversity in the Arabian Gulf: Threats and Conservation. In Biodiversity—The Dynamic Balance of the Planet; InTech Publishing: London, UK, 2014; pp. 297–328. [Google Scholar]
- Ismael, A.A. Coastal Engineering and Harmful Algal Blooms along Alexandria Coast, Egypt. Egypt. J. Aquat. Res. 2014, 40, 125–131. [Google Scholar] [CrossRef] [Green Version]
- El-Askary, H.; Abd El-Mawla, S.H.; Li, J.; El-Hattab, M.M.; El-Raey, M. Change Detection of Coral Reef Habitat Using Landsat-5 TM, Landsat 7 ETM+ and Landsat 8 OLI Data in the Red Sea (Hurghada, Egypt). Int. J. Remote Sens. 2014, 35, 2327–2346. [Google Scholar] [CrossRef]
- Robertson, A.I.; Phillips, M.J. Mangroves as Filters of Shrimp Pond Effluent: Predictions and Biogeochemical Research Needs. Hydrobiologia 1995, 295, 311–321. [Google Scholar] [CrossRef]
- Berktay, A. Environmental Approach and Influence of Red Tide to Desalination Process in the Middle East Region. Int. J. Chem. Environ. Eng. 2011, 2, 183–188. [Google Scholar]
- Al Shehhi, M.R.; Gherboudj, I.; Ghedira, H. Satellites-Based Monitoring of Harmful Algal Blooms for Sustainable Desalination. In Desalination Sustainability—A Technical, Socioeconomic, and Environmental Approach; Elsevier: Amsterdam, The Netherlands, 2017; pp. 341–366. [Google Scholar] [CrossRef]
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The Catastrophic 2008–2009 Red Tide in the Arabian Gulf Region, with Observations on the Identification and Phylogeny of the Fish-Killing Dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- Moufaddal, W.M. Use of Satellite Imagery as Environmental Impact Assessment Tool: A Case Study from the NW Egyptian Red Sea Coastal Zone. Environ. Monit. Assess. 2005, 107, 427–452. [Google Scholar] [CrossRef]
- Al-Azri, A.; Piontkovski, S.; Al-Hashmi, K.; Al-Gheilani, H.; Al-Habsi, H.; Al-Khusaibi, S.; Al-Azri, N. The Occurrence of Algal Blooms in Omani Coastal Waters. Aquat. Ecosyst. Health Manag. 2012, 15, 56–63. [Google Scholar] [CrossRef]
- Karbassi, A.; Abdollahzadeh, E.M.; Attaran-Fariman, G.; Nazariha, M.; Mazaheri-Assadi, M. Predicting the Distribution of Harmful Algal Bloom (HAB) in the Coastal Area of Oman Sea. Nat. Environ. Pollut. Technol. 2017, 16, 753. [Google Scholar]
- Saifullah, S.M.; Mandura, A.S.; Khafaji, A.K. A Platymonas Bloom in Coastal Waters of Jeddah, Saudi Arabia. Pak. J. Bot. 1988, 20, 285–289. [Google Scholar]
- Mikhail, S.K. First Monospecific Bloom of the Harmful Raphidophyte Chattonella antiqua (Hada) Ono in Alexandria Waters Related to Water Quality and Copepod Grazing. Chem. Ecol. 2007, 23, 393–407. [Google Scholar] [CrossRef]
- Al-Hashmi, K.A.; Smith, S.L.; Claereboudt, M.; Piontkovski, S.A.; Al-Azri, A. Dynamics of Potentially Harmful Phytoplankton in a Semi-Enclosed Bay in the Sea of Oman. Bull. Mar. Sci. 2015, 91, 141–166. [Google Scholar] [CrossRef]
- Arnone, R.A.; Ladner, S.; La Violette, P.E.; Brock, J.C.; Rochford, P.A. Seasonal and Interannual Variability of Surface Photosynthetically Available Radiation in the Arabian Sea. J. Geophys. Res. 1998, 103, 7735–7748. [Google Scholar] [CrossRef]
- Chang, J.-H. Global Distribution of Net Radiation According to a New Formula. Ann. Assoc. Am. Geogr. 1970, 60, 340–351. [Google Scholar] [CrossRef]
- Kumar, S.P.; Narvekar, J. Seasonal Variability of the Mixed Layer in the Central Arabian Sea and Its Implication on Nutrients and Primary Productivity. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 1848–1861. [Google Scholar] [CrossRef]
- Shenoi, S.S.C.; Shankar, D.; Gopalakrishna, V.V.; Durand, F. Role of Ocean in the Genesis and Annihilation of the Core of the Warm Pool in the Southeastern Arabian Sea. Mausam 2005, 56, 147–160. [Google Scholar] [CrossRef]
- Weller, R.A.; Baumgartner, M.F.; Josey, S.A.; Fischer, A.S.; Kindle, J.C. Atmospheric Forcing in the Arabian Sea during 1994–1995: Observations and Comparisons with Climatology and Models. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 1961–1999. [Google Scholar] [CrossRef]
- Ahmad, F.; Sultan, S.A.R. Annual Mean Surface Heat Fluxes in the Arabian Gulf and the Net Heat Transport through the Strait of Hormuz. Atmos.-Ocean 1991, 29, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Premkumar, K.; Ravichandran, M.; Kalsi, S.R.; Sengupta, D.; Gadgil, S. First Results from a New Observational System over the Indian Seas. Curr. Sci. 2000, 78, 323–330. [Google Scholar]
- Ramanathan, V.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, Climate, and the Hydrological Cycle. Science 2001, 294, 2119–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahul, P.R.C.; Salvekar, P.S.; Devara, P.C.S. Aerosol Optical Depth Variability over Arabian Sea during Drought and Normal Years of Indian Monsoon. Geophys. Res. Lett. 2008, 35, L22812. [Google Scholar] [CrossRef]
- Dwivedi, R.M.; Raman, M.; Babu, K.N.; Singh, S.K.; Vyas, N.K.; Matondkar, S.G.P. Formation of Algal Bloom in the Northern Arabian Sea Deep Waters during January–March: A Study Using Pooled in Situ and Satellite Data. Int. J. Remote Sens. 2008, 29, 4537–4551. [Google Scholar] [CrossRef]
- Hamza, W.; Enan, M.R.; Al-Hassini, H.; Stuut, J.-B.; de-Beer, D. Dust Storms over the Arabian Gulf: A Possible Indicator of Climate Changes Consequences. Aquat. Ecosyst. Health Manag. 2011, 14, 260–268. [Google Scholar] [CrossRef]
- Vinoj, V.; Satheesh, S.K. Measurements of Aerosol Optical Depth over Arabian Sea during Summer Monsoon Season. Geophys. Res. Lett. 2003, 30, 1263. [Google Scholar] [CrossRef]
- Evan, A.T.; Kossin, J.P.; Chung, C.E.; Ramanathan, V. Arabian Sea Tropical Cyclones Intensified by Emissions of Black Carbon and Other Aerosols. Nature 2011, 479, 94–97. [Google Scholar] [CrossRef]
- Ramanathan, V.; Crutzen, P.J.; Mitra, A.P.; Sikka, D. The Indian Ocean Experiment and the Asian Brown Cloud. Curr. Sci. 2002, 83, 947–955. [Google Scholar]
- Sathyendranath, S.; Stuart, V.; Irwin, B.D.; Maass, H.; Savidge, G.; Gilpin, L.; Platt, T. Seasonal Variations in Bio-Optical Properties of Phytoplankton in the Arabian Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 1999, 46, 633–653. [Google Scholar] [CrossRef]
- Longhurst, A. Seasonal Cycles of Pelagic Production and Consumption. Prog. Oceanogr. 1995, 36, 77–167. [Google Scholar] [CrossRef]
- Ben-David-Zaslow, R.; Henning, G.; Hofmann, D.K.; Benayahu, Y. Reproduction in the Red Sea Soft Coral Heteroxenia fuscescens: Seasonality and Long-Term Record (1991 to 1997). Mar. Biol. 1999, 133, 553–559. [Google Scholar] [CrossRef]
- Labiosa, R.G.; Arrigo, K.R.; Genin, A.; Monismith, S.G.; van Dijken, G. The Interplay between Upwelling and Deep Convective Mixing in Determining the Seasonal Phytoplankton Dynamics in the Gulf of Aqaba: Evidence from SeaWiFS and MODIS. Limnol. Oceanogr. 2003, 48, 2355–2368. [Google Scholar] [CrossRef] [Green Version]
- Alkawri, A.; Gamoyo, M. Remote Sensing of Phytoplankton Distribution in the Red Sea and Gulf of Aden. Acta Oceanol. Sin. 2014, 33, 93–99. [Google Scholar] [CrossRef]
- Gittings, J.A.; Raitsos, D.E.; Racault, M.-F.; Brewin, R.J.W.; Pradhan, Y.; Sathyendranath, S.; Platt, T. Seasonal Phytoplankton Blooms in the Gulf of Aden Revealed by Remote Sensing. Remote Sens. Environ. 2017, 189, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Al-Azri, A.R.; Piontkovski, S.A.; Al-Hashmi, K.A.; Goes, J.I.; Gomes, H.D.R. Recent Outbreaks of Harmful Algal Blooms along the Coast of Oman: Possible Response to Climate Change? In Indian Ocean Tropical Cyclones and Climate Change; Springer: Cham, Switzerland, 2010; pp. 349–357. [Google Scholar]
- Lierheimer, L.J.; Banse, K. Seasonal and Interannual Variability of Phytoplankton Pigment in the Laccadive (Lakshadweep) Sea as Observed by the Coastal Zone Color Scanner. Proc. Indian Acad. Sci.-Earth Planet. Sci. 2002, 111, 163–185. [Google Scholar] [CrossRef] [Green Version]
- Al-Azri, A.R.; Piontkovski, S.A.; Al-Hashmi, K.A.; Goes, J.I.; Gomes, H.D.R.; Glibert, P.M. Mesoscale and Nutrient Conditions Associated with the Massive 2008 Cochlodinium Polykrikoides Bloom in the Sea of Oman/Arabian Gulf. Estuaries Coasts 2014, 37, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, N.; Ramesh, R.; Prakash, S.; Kumar, S. Nitrogen Sources for New Production in the NE Arabian Sea. J. Sea Res. 2011, 65, 265–274. [Google Scholar] [CrossRef]
- Capone, D.G.; Subramaniam, A.; Montoya, J.P.; Voss, M.; Humborg, C.; Johansen, A.M.; Siefert, R.L.; Carpenter, E.J. An Extensive Bloom of the N2-Fixing Cyanobacterium Trichodesmium erythraeum in the Central Arabian Sea. Mar. Ecol. Prog. Ser. 1998, 172, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Schnack-Schiel, S.B.; Niehoff, B.; Hagen, W.; Böttger-Schnack, R.; Cornils, A.; Dowidar, M.M.; Pasternak, A.; Stambler, N.; Stübing, D.; Richter, C. Population Dynamics and Life Strategies of Rhincalanus nasutus (Copepoda) at the Onset of the Spring Bloom in the Gulf of Aqaba (Red Sea). J. Plankton Res. 2008, 30, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Al-Busaidi, S.S.; Al-Rashdi, K.M.; Al-Gheilani, H.M.; Amer, S. Hydrographical Observations during a Red Tide with Fish Mortalities at Masirah Island, Oman. J. Agric. Mar. Sci. 2008, 13, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Sarma, V.; Patil, J.S.; Shankar, D.; Anil, A.C. Shallow Convective Mixing Promotes Massive Noctiluca scintillans Bloom in the Northeastern Arabian Sea. Mar. Pollut. Bull. 2019, 138, 428–436. [Google Scholar] [CrossRef]
- Harrison, P.J.; Furuya, K.; Glibert, P.M.; Xu, J.; Liu, H.B.; Yin, K.; Lee, J.H.W.; Anderson, D.M.; Gowen, R.; Al-Azri, A.R. Geographical Distribution of Red and Green Noctiluca scintillans. Chin. J. Oceanol. Limnol. 2011, 29, 807–831. [Google Scholar] [CrossRef]
- Post, A.F.; Dedej, Z.; Gottlieb, R.; Li, H.; Thomas, D.N.; El-Absawi, M.; El-Naggar, A.; El-Gharabawi, M.; Sommer, U. Spatial and Temporal Distribution of Trichodesmium spp. in the Stratified Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 2002, 239, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Kawamura, H.; Luis, A.J. Short-Term Variability of Phytoplankton Blooms Associated with a Cold Eddy in the Northwestern Arabian Sea. Remote Sens. Environ. 2002, 81, 82–89. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Pradhan, Y.; Brewin, R.J.W.; Stenchikov, G.; Hoteit, I. Remote Sensing the Phytoplankton Seasonal Succession of the Red Sea. PLoS ONE 2013, 8, e64909. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; El-Askary, H.; Qurban, M.A.; Proestakis, E.; Garay, M.J.; Kalishnikova, O.V.; Amiridis, V.; Gkikas, A.; Marinou, E.; Piechota, T. An Assessment of Atmospheric and Meteorological Factors Regulating Red Sea Phytoplankton Growth. Remote Sens. 2018, 10, 673. [Google Scholar] [CrossRef] [Green Version]
- Kheireddine, M.; Ouhssain, M.; Claustre, H.; Uitz, J.; Gentili, B.; Jones, B.H. Assessing Pigment-Based Phytoplankton Community Distributions in the Red Sea. Front. Mar. Sci. 2017, 4, 132. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, Z.A.; Mesaad, I. First Report on Noctiluca Scintillans Blooms in the Red Sea off the Coasts of Saudi Arabia: Consequences of Eutrophication. Oceanologia 2007, 49, 337–351. [Google Scholar]
- Li, W.; El-Askary, H.; ManiKandan, K.P.; Qurban, M.A.; Garay, M.J.; Kalashnikova, O.V. Synergistic Use of Remote Sensing and Modeling to Assess an Anomalously High Chlorophyll-a Event during Summer 2015 in the South Central Red Sea. Remote Sens. 2017, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Racault, M.-F.; Raitsos, D.E.; Berumen, M.L.; Brewin, R.J.W.; Platt, T.; Sathyendranath, S.; Hoteit, I. Phytoplankton Phenology Indices in Coral Reef Ecosystems: Application to Ocean-Color Observations in the Red Sea. Remote Sens. Environ. 2015, 160, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, R.K.; Chauhan, P.; Nayak, S.R. Inter-Annual Variability of Phytoplankton Blooms in the Northern Arabian Sea during Winter Monsoon Period (February–March) Using IRS-P4 OCM Data. Indian J. Geo-Mar. Sci. 2005, 34, 163–173. [Google Scholar]
- Banzon, V.F.; Evans, R.E.; Gordon, H.R.; Chomko, R.M. SeaWiFS Observations of the Arabian Sea Southwest Monsoon Bloom for the Year 2000. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 189–208. [Google Scholar] [CrossRef]
- Ranjha, R.; Tjernström, M.; Semedo, A.; Svensson, G.; Cardoso, R.M. Structure and Variability of the Oman Coastal Low-Level Jet. Tellus A Dyn. Meteorol. Oceanogr. 2015, 67, 25285. [Google Scholar] [CrossRef]
- Stihl, A.; Sommer, U.; Post, A.F. Alkaline Phosphatase Activities among Populations of the Colony-forming Diazotrophic Cyanobacterium Trichodesmium spp. (Cyanobacteria) in the Red Sea. J. Phycol. 2001, 37, 310–317. [Google Scholar] [CrossRef]
- Dwivedi, R.M.; Raman, M.; Parab, S.; Matondkar, S.G.P.; Nayak, S. Influence of Northeasterly Trade Winds on Intensity of Winter Bloom in the Northern Arabian Sea. Curr. Sci. 2006, 90, 1397–1406. [Google Scholar]
- Brock, J.C.; Sathyendranath, S.; Platt, T. Biohydro-Optical Classification of the Northwestern Indian Ocean. Mar. Ecol. Prog. Ser. 1998, 165, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.; Veizer, J. Water Balance of the Indus River Basin and Moisture Source in the Karakoram and Western Himalayas: Implications from Hydrogen and Oxygen Isotopes. J. Geophys. Res. Atmos. 2002, 107, ACH 9-1–ACH 9-12. [Google Scholar] [CrossRef]
- Shrestha, S.; Nepal, S. Water Balance Assessment under Different Glacier Coverage Scenarios in the Hunza Basin. Water 2019, 11, 1124. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Bano, R.; Ansari, K.; Shaikh, M.N. Hydrology of Upper Indus Basin. In Proceedings of the 4th International Conference on Energy, Environment and Sustainable Development 2016, Jamshoro, Pakistan, 1–3 November 2016. [Google Scholar]
- Al-Shehhi, M.R.; Nelson, D.; Farzanah, R.; Alshihi, R.; Salehi-Ashtiani, K. Characterizing Algal Blooms in a Shallow & a Deep Channel. Ocean Coast. Manag. 2021, 213, 105840. [Google Scholar]
- Nassar, M.Z. Nutrients and Phytoplankton Distribution in the Coastal Waters of Aqaba Gulf, Red Sea, Egypt. Egypt. J. Aquat. Res. 2007, 33, 133–151. [Google Scholar]
- Prakash, S.; Ramesh, R.; Sheshshayee, M.S.; Dwivedi, R.M.; Raman, M. Quantification of New Production during a Winter Noctiluca scintillans Bloom in the Arabian Sea. Geophys. Res. Lett. 2008, 35, L08604. [Google Scholar] [CrossRef]
- Basu, S.; Deobagkar, D.D.; Matondkar, S.G.P.; Furtado, I. Culturable Bacterial Flora Associated with the Dinoflagellate Green Noctiluca Miliaris during Active and Declining Bloom Phases in the Northern Arabian Sea. Microb. Ecol. 2013, 65, 934–954. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Rafeeq, M.; Smitha, B.R.; Padmakumar, K.B.; Thomas, L.C.; Sanjeevan, V.N.; Prakash, P.; Raman, M. Species Identification of Mixed Algal Bloom in the Northern Arabian Sea Using Remote Sensing Techniques. Environ. Monit. Assess. 2015, 187, 51. [Google Scholar] [CrossRef]
- Al-Hashmi, K.; Sarma, Y.V.B.; Claereboudt, M.; Al-Azri, A.R.; Piontkovski, S.A.; Al-Habsi, H. Phytoplankton Community Structure in the Bay of Bandar Khyran, Sea of Oman with Special Reference to Harmful Algae. Int. J. Mar. Sci. 2012, 2, 31–42. [Google Scholar]
- Baliarsingh, S.K.; Lotliker, A.A.; Sudheesh, V.; Samanta, A.; Das, S.; Vijayan, A.K. Response of Phytoplankton Community and Size Classes to Green Noctiluca Bloom in the Northern Arabian Sea. Mar. Pollut. Bull. 2018, 129, 222–230. [Google Scholar] [CrossRef]
- Sarangi, R.K. Observation of Algal Bloom in the Northwest Arabian Sea Using Multisensor Remote Sensing Satellite Data. Mar. Geod. 2012, 35, 158–174. [Google Scholar] [CrossRef]
- Basu, S.; Matondkar, S.G.P.; Furtado, I. Enumeration of Bacteria from a Trichodesmium spp. Bloom of the Eastern Arabian Sea: Elucidation of Their Possible Role in Biogeochemistry. J. Appl. Phycol. 2011, 23, 309–319. [Google Scholar] [CrossRef]
- Jyothibabu, R.; Karnan, C.; Jagadeesan, L.; Arunpandi, N.; Pandiarajan, R.S.; Muraleedharan, K.R.; Balachandran, K.K. Trichodesmium Blooms and Warm-Core Ocean Surface Features in the Arabian Sea and the Bay of Bengal. Mar. Pollut. Bull. 2017, 121, 201–215. [Google Scholar] [CrossRef]
- Desa, E.S.; Suresh, T.; Matondkar, S.G.P.; Desa, E.; Goes, J.; Mascarenhas, A.; Parab, S.G.; Shaikh, N.; Fernandes, C.E.G. Detection of Trichodesmium Bloom Patches along the Eastern Arabian Sea by IRS-P4/OCM Ocean Color Sensor and by in-Situ Measurements. Indian J. Geo-Mar. Sci. 2005, 34, 374–386. [Google Scholar]
- Krishnan, A.A.; Krishnakumar, P.K.; Rajagopalan, M. Trichodesmium erythraeum (Ehrenberg) Bloom along the Southwest Coast of India (Arabian Sea) and Its Impact on Trace Metal Concentrations in Seawater. Estuar. Coast. Shelf Sci. 2007, 71, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, B.R.; Deepthi, T.; Ashwini, S.; Shylini, S.K.; Kumarswami, M.; Kumar, S.; Lotliker, A.A. Spring Inter Monsoon Algal Blooms in the Eastern Arabian Sea: Shallow Marine Encounter off Karwar and Kumbla Coast Using a Hyperspectral Radiometer. Int. J. Earth Sci. Eng. 2010, 3, 827–832. [Google Scholar]
- Padmakumar, K.B.; Smitha, B.R.; Thomas, L.C.; Fanimol, C.L.; SreeRenjima, G.; Menon, N.R.; Sanjeevan, V.N. Blooms of Trichodesmium erythraeum in the South Eastern Arabian Sea during the Onset of 2009 Summer Monsoon. Ocean Sci. J. 2010, 45, 151–157. [Google Scholar] [CrossRef]
- Touliabah, H.E.; El-Kheir, W.S.A.; Kuchari, M.G.; Abdulwassi, N.I.H. Phytoplankton Composition at Jeddah Coast-Red Sea, Saudi Arabia in Relation to Some Ecological Factors. JKAU Sci. 2010, 22, 115–131. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, N.; Singh, A.; Prakash, S.; Ramesh, R.; Raman, M.; Sheshshayee, M.S.; Shetye, S. First Direct Measurements of N2 Fixation during a Trichodesmium Bloom in the Eastern Arabian Sea. Glob. Biogeochem. Cycles 2011, 25, GB4014. [Google Scholar] [CrossRef]
- Raghavan, B.R.; Raman, M.; Chauhan, P.; Kumar, B.S.; Shylini, S.K.; Mahendra, R.S.; Nayak, S.R. Summer Chlorophyll-a Distribution in Eastern Arabian Sea off Karnataka-Goa Coast from Satellite and in-Situ Observations. In Proceedings of the Remote Sensing of the Marine Environment; International Society for Optics and Photonics, Goa, India, 15–17 November 2006; Volume 6406, p. 64060W. [Google Scholar]
- Roy, R.; Pratihary, A.; Narvenkar, G.; Mochemadkar, S.; Gauns, M.; Naqvi, S.W.A. The Relationship between Volatile Halocarbons and Phytoplankton Pigments during a Trichodesmium Bloom in the Coastal Eastern Arabian Sea. Estuar. Coast. Shelf Sci. 2011, 95, 110–118. [Google Scholar] [CrossRef]
- Häse, C.; Al-Qutob, M.; Dubinsky, Z.; Ibrahim, E.A.; Lazar, B.; Stambler, N.; Tilzer, M.M. A System in Balance?? Implications of Deep Vertical Mixing for the Nitrogen Budget in the Northern Red Sea, Including the Gulf of Aqaba (Eilat). Biogeosci. Discuss. 2006, 3, 383–408. [Google Scholar]
- Tang, W.; Cerdán-García, E.; Berthelot, H.; Polyviou, D.; Wang, S.; Baylay, A.; Whitby, H.; Planquette, H.; Mowlem, M.; Robidart, J. New Insights into the Distributions of Nitrogen Fixation and Diazotrophs Revealed by High-Resolution Sensing and Sampling Methods. ISME J. 2020, 14, 2514–2526. [Google Scholar] [CrossRef]
- Munir, S.; Naz, T.; Burhan, Z.; Siddiqui, P.J.A.; Morton, S.L. First Report of the Athecate, Chain Forming Dinoflagellate Cochlodinium fulvescens (Gymnodiniales) from Pakistan. Pak. J. Bot. 2012, 44, 2129–2134. [Google Scholar]
- Bauman, A.G.; Burt, J.A.; Feary, D.A.; Marquis, E.; Usseglio, P. Tropical Harmful Algal Blooms: An Emerging Threat to Coral Reef Communities? Mar. Pollut. Bull. 2010, 60, 2117–2122. [Google Scholar] [CrossRef]








| Region | Location | SWM | NEM | ||
|---|---|---|---|---|---|
| Months | Cause | Months | Cause | ||
| Arabian Sea | 1 | August–November | Upwelling and advection | ||
| 4 | August–September October November | Upwelling and weak winds Upwelling Mixing | May | Northward advection of blooms | |
| 5 | June | Mixing (entrainment nutrients) | May November December | Advection of nutrients from Oman and solar radiation Mixing (entrainment nutrients) Mixing and advection of nutrients from Arabian coast | |
| 2 | October | Southward advection of nutrients | February–May | Highest solar radiation, southward advection of nutrients, and strong wind | |
| 3 | October–Dec | Upwelling and effects of the Indus river | February–May | Wind, northward advection, and effect of Indus river | |
| Sea of Oman | 6 | Aug–October | Advection from Arabian Sea and upwelling | January–April | Upwelling, solar radiation, northward advection, and dust storms |
| Arabian Gulf | 7 | October–November | Solar radiation, wind and dust storms, and warm air temperatures | January–May | Advection from Sea of Oman, dust storms, and warm air temperatures |
| 8 | June–August | Dust storms and organic nutrients | March–May | Low wind, stable water column, and discharge of Shatt Al-Arab | |
| Red Sea | 9 | June–August | Northward advection of nutrients, upwelling, and solar radiation | February–Mar | Upwelling, wind, and dust storms |
| 10 | February–Mar | Upwelling, wind, and dust storms | |||
| Anthropogenic Activities | Examples | Ref. |
|---|---|---|
| Industrial effluents |
| [50,51] |
| [52,53] | |
| [4,42,43,46,54,55,56,57] | |
| Ship waste |
| [3,43,53,58] |
| Reclamation and urban coastal projects |
| [46,48] |
| Sunlight Effect | Influence | Ref. |
|---|---|---|
| Photosynthetic fixation |
| [59,60] |
| Surface mixed-layer depth |
| [61] |
| Temperature profile |
| [59,62] |
| Upwelling Effect | Season | Region | Ref. |
|---|---|---|---|
| Decrease in mixed-layer depth | SWM |
| [25,30,75,82] |
| NEM |
| [25,82] | |
| Supply of nutrients (phosphate, nitrate, and silicate) to the mixed layer | SWM |
| [28,30,75,76,78,83] |
| NEM |
| [28,54,84,85] | |
| Cooling seawater | SWM |
| [28,76,85] |
| NEM |
| [30] |
| Flow Patterns | Season | Region | Ref. |
|---|---|---|---|
| Mesoscale eddies | SWM | ||
| NEM |
| [27,28,89,96] | |
| Cyclonic and anti-cyclonic large eddies | SWM |
| [25,28,58,74] |
| NEM | |||
| Local cyclonic eddies | SWM | ||
| NEM |
| [90,92,94] | |
| Water currents | SWM |
| [3,25,28,37,55,90,95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shehhi, M.R.; Abdul Samad, Y. Identifying Algal Bloom ‘Hotspots’ in Marginal Productive Seas: A Review and Geospatial Analysis. Remote Sens. 2022, 14, 2457. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14102457
Al-Shehhi MR, Abdul Samad Y. Identifying Algal Bloom ‘Hotspots’ in Marginal Productive Seas: A Review and Geospatial Analysis. Remote Sensing. 2022; 14(10):2457. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14102457
Chicago/Turabian StyleAl-Shehhi, Maryam R., and Yarjan Abdul Samad. 2022. "Identifying Algal Bloom ‘Hotspots’ in Marginal Productive Seas: A Review and Geospatial Analysis" Remote Sensing 14, no. 10: 2457. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14102457