1. Introduction
Water clarity, or transparency, is an important characteristic of marine ecosystem health, affecting the primary resource (light) required by photosynthetic organisms. Ecosystems such as coral reefs and seagrass meadows are built by photosynthetic organisms, and are therefore highly sensitive to changes in water clarity [
1]. Recently, ocean color remote sensing techniques have complemented field sampling to monitor water clarity in coral reefs. Ocean color remote sensing allows large scale, synoptic water clarity monitoring where
in situ physical sampling is difficult and costly [
2,
3]. Satellite sensors provide spectral radiometric measurements of the color of the ocean that can be directly related to the relative concentrations of optically-active constituents, such as phytoplankton, dissolved organic matter or suspended particulate matter [
4].
Empirical and physics-based algorithms relate sensor-observed remote-sensing reflectance signals to
in situ marine components. The radiative transfer problem of optically deep waters has been widely researched, with deep-water ocean color algorithms meeting NASA mission required accuracies for water-leaving radiance and chlorophyll-a retrievals, e.g., [
5,
6,
7,
8]. On the other hand, deriving reliable ocean color products for optically shallow water masses, where light reflected from the seafloor contributes to the net water-leaving radiance, is more challenging and requires specialized algorithms. Water clarity monitoring of optically shallow waters using ocean color imagery data requires an understanding of the effects of bottom reflectance on the surface reflectance signal.
Initial efforts in the development of shallow water inversion algorithms focused primarily on the simultaneous retrieval of bathymetry and bottom cover. Less attention was given to the derivation of the inherent optical properties (IOPs) of the water column [
9,
10,
11,
12,
13]. More recently, effort has focused on the development of ocean color inversion algorithms for IOP retrievals in optically shallow waters [
14,
15,
16,
17]. One example is the newly developed Shallow Water Inversion Model (SWIM) algorithm, currently implemented as an evaluation product in NASA’s ocean color processing code, L2GEN [
17]. SWIM is based on the shallow water optical model of Lee
et al. [
18]; however, the SWIM algorithm does not retrieve water depth and bottom reflectance as free parameters. Instead, estimates of water column depth and benthic albedo (reflectance) are supplied to SWIM as ancillary data inputs. The current implementation of SWIM for the Great Barrier Reef (GBR), Australia, has been developed with the requirement of two specific regional input datasets, bathymetry and benthic albedo: Reliable bathymetry data at 100 m spatial resolution are available over the full extent of the GBR [
19]. Prior to this study, an existing benthic biodiversity database [
20] was used to derive the bottom reflectance signatures for a simple two-component “light” and “dark” reflectance map [
21].
However, the optimal parameterization of bottom reflectance in shallow water inversion models is still not well constrained, particularly with respect to the spectral signature and number of required spectral classes. There remains a need to resolve spectral separability for current ocean color sensors to optimize bottom reflectance parameterization, and thus IOP retrievals in shallow water inversion models.
Quantifying the bottom reflectance contribution to the remote-sensing reflectance signals is challenging due to heterogeneous bottom cover and differences in spatial and spectral resolutions of common ocean color sensors. Current ocean color satellite sensors have limited capabilities to resolve bottom types or communities, such as sand, seagrass, algae or coral, due to the limited number and placement of their spectral bands [
22]. Most sensors have 6–15 spectral bands in the 400–1050 nm optical range, spatial pixel resolutions ranging from 250 m to 1.1 km and spatial swath extents of 1000s of kilometers. Whilst planned next generation satellite sensors with improved spectral and/or spatial resolution, such as the Pre-Aerosol Cloud and ocean Ecosystem (PACE) and the Ocean Land Color Instrument (OLCI) (Sentinel-3) missions [
23], may be able to better differentiate bottom cover spectral signatures, data acquisition from such sensors is still likely to be coarse, with pixels sizes of 300 m to 1 km in size. Moderate resolution satellite sensors, such as the Moderate Resolution Imaging Spectroradiometer (MODIS), Medium Resolution Imaging Spectrometer (MERIS) and Sea-Viewing Wide Field-of-View Sensor (SeaWiIFS), are currently used by the satellite remote sensing community due to ease of data accessibility and large spatial (global) and temporal (daily) coverage. Numerous previous studies have assessed the spectral separability of different bottom types based on pure endmembers (single organisms or substrate types) within a small area (<1 m
2) [
24,
25,
26,
27,
28,
29]. Only a few such investigations, however, have assessed the impact of bottom type mixtures on the remote-sensing reflectance signal [
30,
31]. Those studies have focused on higher spatial resolution sensors with pixel sizes of <50 m. Data from moderate resolution sensors are represented by 6-to-7 visible bands (
Table 1) and relatively large pixel sizes (1 km × 1 km), which typically contain a mixture of bottom types in one pixel. Therefore, it is particularly important to assess the impact of mixed substrate pixels on their spectral separability, rather than analyzing single bottom covers.
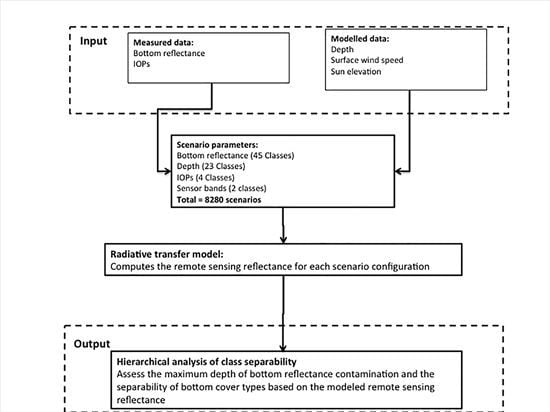
The primary objective of this study therefore was to determine a reliable and efficient approach to discern the optimal bottom cover spectral parameterization for shallow water inversion algorithms. Specifically, we have focused on shallow water inversion algorithms applied to moderate resolution ocean color remote sensing of coral reef environments by: (1) determining threshold depths at which the bottom reflectance signal of individual and mixed bottom types can contribute to the remote-sensing reflectance signal; and (2) determining the number of bottom spectral signatures required to accurately characterize bottom reflectance in shallow water inversion models. We applied the methodology to the MODIS and SeaWiFS spectral bands. Due to project restraints, MERIS data were not used in this study, however the methods are similarly applicable.
Table 1.
Assessed band center and bandwidths (nm) used for the statistical analysis of spectral separability and detectability of bottom types.
Table 1.
Assessed band center and bandwidths (nm) used for the statistical analysis of spectral separability and detectability of bottom types.
| Sensor | Band Center (Band Width) (units: nm) |
|---|
| MODIS | 412.5 (15) | 443 (10) | 488 (10) | 531 (10) | 551 (10) | 667 (10) | 677.5 (10) |
| SeaWiFS | 412 (20) | 443 (20) | 490 (20) | 510 (20) | 555 (20) | 670 (20) | |
4. Discussion
This study assessed the influence of bottom reflectance on the spectrally-averaged Rrs signal measured by the moderate resolution SeaWiFS and MODIS sensors in optically shallow waters of coral reef environments. The results showed: (i) that there was no significant (Rrscorr < 0.0005 sr−1) influence of bottom reflectance on the Rrs signal for depths >19 m for either sensor; and (ii) that the assessed bottom cover classes can be amalgamated into two distinct functional groups, “light” and “dark”, based on the modeled Rrs surface reflectance signals. Only Rrs spectra dominated by light sand and its mixtures can be clearly discriminated from other bottom cover types typically found in coral reef waters.
SeaWiFS and MODIS
Rrs data are routinely used to derive IOPs and a number of IOP-based geophysical products such as Chla and the diffuse attenuation coefficient (
Kd). Light reflected off the seafloor in optically shallow waters contaminates the sensor-observed
Rrs signal and subsequently causes errors in the derived IOPs. The recently-developed semi-analytical SWIM algorithm was specifically devised to improve IOP retrievals in optically shallow coral reef waters, such as the GBR. An essential input component of the SWIM algorithm is a bottom reflectance map [
17]. To construct a bottom reflectance map, it is essential to know the number of distinct spectral classes to be mapped and which spectra best represent these classes [
17]. Further, it is useful to know in which geographic areas bottom reflectance is most likely to contaminate
Rrs and therefore needs to be included in the bottom reflectance map. To address this, we determined the maximum geometric depth at which bottom reflectance may be detectable under different IOP/water clarity scenarios.
The maximum depth of bottom detectability for clear reefal waters of the GBR was determined to be 17 m and 19 m for spectrally-averaged MODIS band SeaWiFS bands, respectively. However, the depth of bottom detectability was reduced substantially in highly attenuating, inshore waters. Hence the SWIM algorithm may not need to account for bottom reflectance where the water column depth exceeds 19 m. We found bottom reflectance from seagrass, a relatively dark substrate, had no influence on spectrally-averaged
Rrs at depths exceeding 15 m for MODIS bands and depths exceeding 11 m for SeaWiFS. Seagrass occurrence is prevalent in coral reef waters and has been recorded down to depths of 61 m in the GBR [
54]. In Estuarine waters, which are dominated by terrigenous runoff, particularly in the summer wet season, bottom reflectance contamination was found to be minimal and undetectable in waters >5 m. In Coastal water types, darker bottom covers such as seagrass were also undetectable at depths >5 m.
The minor differences in the maximum depth of bottom detectability between MODIS and SeaWiFS may be explained by the placement of their spectral bands. For example, for Reef Waters using the light sand bottom spectra, SeaWiFS provided a slightly deeper maximum depth than MODIS (19 m
vs. 17 m), which is likely due to the placement and width of the assessed bands. The differences in the bands 490/488 (SeaWiFS band 3 and MODIS/Aqua band 10, respectively) and 555/551 (SeaWiFS band 5 and MODIS/Aqua band 12, respectively) result in different radiance retrievals for these blue-green bands [
55], which may have caused the minor differences in maximum depth of detectability. Further, the minor difference in maximum depth of bottom reflectance detectability might be due to band-averaging, as MODIS has two red bands compared to one for SeaWiFS. In addition, our study used 2 m depth increments, thus the real difference in maximum depth of bottom reflectance detectability lies within a 0–2 m depth range. Even a 2 m depth difference in a 1km × 1 km pixel is relatively minor and is not expected to make much difference to IOP retrievals using semi-analytical inversion algorithms.
We focused on the band-averaged maximum depth of bottom reflectance detectability to investigate to which depth MODIS and SeaWiFS satellite sensors could detect bottom signals affecting shallow water inversion models. We selected a cutoff threshold of 0.00005 sr−1, which was 2% of the maximum, band-averaged, modeled remote sensing reflectance, 0.025 sr−1. Anything below this threshold was considered noise. Therefore, one could argue that no signal from the bottom was recorded below this threshold. However, a minimal influence of benthic albedo was detected at the red bands (>650 nm), where pure water absorption is high. The bottom reflectance contribution was primarily detected in bands at 488 nm, 531 nm and 551 nm for modeled MODIS Rrs and at 490 nm, 510 nm and 555 nm for modeled SeaWiFS Rrs.
The four optical environments used in this study are defined on the basis of chlorophyll, suspended matter and CDOM, rather than on the optical properties themselves. We acknowledge that the simulations of the optical properties are computed within HE5, using conversions to absorption, scattering and backscattering, and therefore may not be always appropriate in coastal waters.
Further, it should be noted that at the resolution of MODIS and SeaWiFS, one would expect mixed depth pixels, as well as mixed bottom types. This might lead to increasing or decreasing detectability and separability of bottom types and thus lead to uncertainties in IOP retrievals.
However, to date there are no studies known to the authors that have ascertained the maximum depth at which MODIS or SeaWiFS-observed Rrs are contaminated by benthic reflectance despite these moderate resolution sensors being commonly used in near-coastal waters by the international scientific community. Some recently developed ocean color shallow water inversion models that retrieve IOPs, such as SWIM, require input of bottom reflectance parameters as model input. Hence, determining the maximum depth of bottom detection at moderate resolution sensor bands is essential to the implementation of shallow water inversion models to coral reef ecosystems.
Here, we presented the maximum depth of bottom reflectance contribution to spectrally-averaged
Rrs for light sand and seagrass spectra only. We found these to represent two contrasting groups in coral reef waters, light
versus dark substrates, based on their average spectral reflectance. Seagrass best represented the dark spectra group for the GBR as seagrass meadows can be thick and extensive there. Besides being the most common bottom cover of the dark spectral group in the GBR, seagrass is also closest to the average spectra of the dark spectral group. In the GBR, seagrass accounts for an estimated 40,000 km
2 of bottom cover [
56] compared to coral reef and algae cover of ~24,158 km
2 [
57], with the remaining ~280,242 km
2 (81%) of the GBR Marine Park comprising primarily sand and mud.
Clustering analysis showed that a two-cluster bottom reflectance input configuration, light and dark, is sufficient for parameterizing a shallow water inversion algorithm for MODIS and SeaWiFS sensors. Assessment of spectral uniqueness based on clustering showed that more clusters resulted in weak cluster structures and misclassified bottom types. Using several spectral samples for each bottom reflectance class allowed us to examine whether particular bottom classes might be ambiguously assigned to a specific cluster and hence misclassified. Modeled
Rrs signals at MODIS bands assigned to two primary clusters allowed more consistent grouping of the individual bottom reflectances, with less bottom classes assigned to an “intermediary” cluster group, than at SeaWiFS bands. The intermediary cluster group contained bottom classes that could not be clearly assigned to C1 (dark) or C2 (light) because some of the five (ten for coral) spectral samples from one bottom class were assigned to C1 while others were assigned to C2. Using SeaWiFS bands, the majority of sand and rubble classes could not be clearly assigned to C1 or C2 as their spectral signatures lay between C1 and C2 (not as light as light sand but also not as dark as seagrass or similar). For either sensor, there was no bottom reflectance detected from seagrass for the Coastal or Estuarine optical scenarios, where the water is turbid, even at a shallow depth of 5 m. However, bottom contamination from light sand was still recorded in coastal waters by MODIS. The results provide insight into the optimal substrate clustering for bottom reflectance parameterization in shallow water models. The endmember and average spectra for the light and dark clusters are presented in the
Appendix.
To date, there have been no bottom cover spectral reflectance studies focusing on spectral separability or spectral uniqueness of bottom reflectance spectra at MODIS or SeaWiFS spectral and spatial resolutions known to the authors. Indeed, most comparative studies of bottom reflectance in shallow waters have focused on habitat classification mapping, e.g., [
26,
38,
58,
59,
60]) that requires a greater level of spatial and spectral detail. Generally, research on substrate spectral uniqueness has been undertaken using sensors with higher spatial resolution (pixel size < 50 m and mostly < 4 m) as they are commonly used to map benthic habitat or bathymetry at higher resolution, e.g., [
15,
28,
29,
61,
62]). The spatial area imaged by these sensors is typically much smaller than the scale of larger coral reef ecosystems such as the GBR. Most high spatial resolution multi- and hyperspectral satellite-borne sensors do not have the temporal or spatial coverage provided by MODIS and SeaWiFS. Indeed, the broad swath and regular repeat orbits afforded by MODIS and SeaWiFS are needed to monitor and manage the ecosystem health of the GBR waters on a near-daily basis.
Higher resolution sensors are typically able to discern smaller objects and image pixels often contain signals from a single substrate class. These smaller objects cannot be distinguished by MODIS or SeaWiFS satellite sensors, as image pixels frequently contain signals from a mixture of substrate types. In order for a homogeneous bottom cover to contribute to sensor-observed Rrs, its size has to be larger than several pixels in a specific satellite image. We made the assumption that, if the bottom cover extent was smaller than the pixel size, the signal detected represented the average brightness of all bottom covers in that pixel. Nevertheless, smaller percentages of particular types of bottom cover, such as small patches of sand between extensive seagrass beds, may be detectable if their reflectance signal dominates a particular pixel. MODIS and SeaWiFS have a coarser spatial resolution than most of the commonly used higher resolution satellite sensors (such as IKONOS, WorldView2, etc.). Thus, bottom covers considered in this study generally occur on spatial scales > 1000 m and are not based on specific species per habitat classification, but rather classified into broader bottom classes, such as algae. A number of pure endmember bottom spectra were combined into mixed bottom types most commonly observed in the GBR at MODIS and SeaWiFS scales.
From an ocean color perspective, we may consider the GBR to be divided into three distinct zones based on water depth and geological features: (1) an inner shelf zone with a depth range of 0–20 m dominated by terrigenous sediment; (2) a middle shelf zone with a depth range of 20–40 m of mixed carbonate-siliciclastic sediment; and (3) an outer shelf zone with a depth range of 40–90 m of carbonate-dominated sediment [
63,
64]. The maximum depth of bottom contamination of 19 m found in this study corresponds primarily to the inner shelf region of 0–20 m. This region, with a width of <60 km, is therefore of primary concern for benthic contamination in ocean color algorithms. Because of resuspension and other processes, this is also the zone where optically complex ocean color remote sensing challenges are the greatest. However, our results showed that the most significant bottom contamination is recorded from light (carbonate) sand, which is mainly found in the middle and outer shelf zones of the GBR [
65]. Hence, this study suggests that the primary areas of concern for benthic contamination of the
Rrs signal may be shallow waters adjacent to coral reefs on the mid- to outer shelf of the GBR, rather than the shallow inner shelf region.