World’s Largest Macroalgal Blooms Altered Phytoplankton Biomass in Summer in the Yellow Sea: Satellite Observations
Abstract
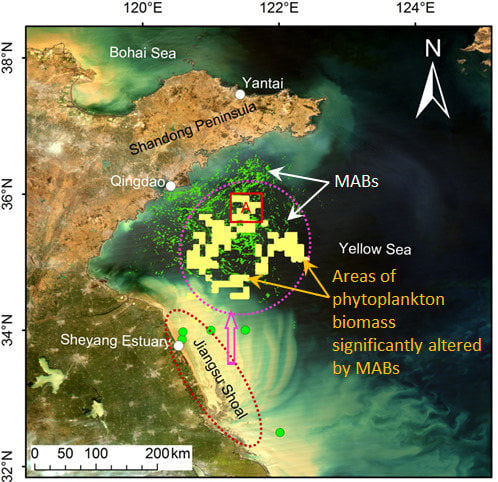
:1. Introduction

2. Data and Methods
2.1 MODIS Level-1 and Level-2 Products
2.2. Removal of Macroalgae-Contaminated Pixels
- (1)
- D0 of Chl-a (µg/L) was first mapped to an equidistant cylindrical projection (D1) using nearest neighbor re-sampling through the software of SeaDAS.
- (2)
- D1 was averaged to D2 by using a moving window of 9 × 9 pixels.
- (3)
- D3 was derived as D1–D2.
- (4)
- Pixels in D3 with their values larger than an optimal threshold (0.5 µg/L) were regarded as macroalgae-contaminated pixels (D4) (see Supplement I for the threshold setting).
- (5)
- D4 was excluded in D1 to generate the water-column Chl-a (D5).
- (6)
- D5 was binned to 9 km × 9 km resolution (D6) to make them consistent with the standard NASA Level-3 product.

2.3. MODIS and SeaWiFS Level-3 Chl-a Products
3. Results
3.1. Impacts of Floating Macroalgae on the Standard MODIS Chl-a Product


3.2. Monthly Chl-a for June and July after the Removal of Macroalgae-Contaminated Pixels



4. Discussion
4.1. Increase in Phytoplankton Biomass in the Bloom Region

4.2. Nutrient Competition between Macroalgae and Phytoplankton

| Year (yyyy) | 2008 | 2009 * | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 * |
|---|---|---|---|---|---|---|---|---|
| Landing date (day/month) | 28/6 | 14/7 | 27/6 | 6/7 | 28/6 | 30/6 | 28/6 | 1/7 |
| Macroalgae | Phytoplankton |
|---|---|
| Mm∙ww, biomass (wet weight), kg: 1 × 109 Rm∙wd, ratio of wet weight to dry weight: 5 Cm∙N, nitrogen content, % (dry weight): 1.5 | A, area with decrease of Chl-a, km2: 9 × 103 WD, water depth with decrease of Chl-a, m: 10 DChl-a, decrease of Chl-a, mg/m3: 0.5 Rbc, ratio of biomass (dry weight) to Chl-a: 130 Cp∙N, nitrogen content, % (dry weight): 3 TO, turn over cycles in a month: 10 |
| Mm∙N, nitrogen in macroalgae, kg: 3 × 106 (Mm∙N = Mm∙ww × (Cm∙N/100)/Rm∙wd) | Mp∙N, nitrogen in phytoplankton, kg: 1.755 × 106 (Mp∙N = A × WD × DChl-a × Rbc × (Cp∙N/100) × TO) |
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, D.A.; Arvanitidis, C.; Blight, A.J.; Chatzinikolaou, E.; Guy-Haim, T.; Kotta, J.; Orav-Kotta, H.; Queiros, A.M.; Rilov, G.; Somerfield, P.J.; et al. Macroalgal blooms alter community structure and primary productivity in marine ecosystems. Glob. Chang. Biol. 2014, 20, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, M.X. Origin and offshore extent of floating algae in Olympic sailing area. Eos Trans. AGU 2008, 89, 302–303. [Google Scholar] [CrossRef]
- Hu, C.; Li, D.; Chen, C.; Ge, J.; Muller-Karger, F.E.; Liu, J.; Yu, F.; He, M. On the recurrent Ulva Prolifera blooms in the Yellow Sea and East China Sea. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Xing, Q.; Loisel, H.; Schmitt, F.; Shi, P.; Liu, D.; Keesing, J. Detection of the green tide at the Yellow Sea and tracking its wind-forced drifting by remote sensing. In Proceedings of the 2009 EGU General Assembly, Vienna, Austria, 19–24 April 2009.
- Xing, Q.; Tosi, L.; Braga, F.; Gao, X.; Gao, M. Interpreting the progressive eutrophication behind the world’s largest macroalgal blooms with water quality and ocean color data. Nat. Hazards 2015, 78, 7–21. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Bao, X.; Zhao, L. Economic cost of an algae bloom cleanup in China’s 2008 Olympic sailing venue. Eos Trans. AGU 2009, 90, 238–239. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zheng, X.; Shi, P.; Hao, J.; Yu, D.; Liang, S.; Liu, D.; Zhang, Y. Monitoring “Green Tide” in the Yellow Sea and the East China Sea using multi-temporal and multi-source remote sensing images. Spectrosc. Spect. Anal. 2011, 31, 1644–1647. (in Chinese). [Google Scholar]
- Zheng, X.; Xing, Q.; Shi, P.; Li, L. Numerical simulation of the 2008 green tide in the Yellow Sea. Mar. Sci. 2011, 35, 82–87. [Google Scholar]
- Smith, D.W.; Horne, A.J. Experimental measurement of resource competition between planktonic microalgae and macroalgae (seaweeds) in mesocosms simulating the San Francisco Bay-Estuary, California. Hydrobiologia 1988, 159, 259–268. [Google Scholar] [CrossRef]
- Fong, P.; Donohoe, R.M.; Zedler, J.B. Competition with macroalgae and benthic cyanobacterial mats limits. Mar. Ecol. Prog. Ser. 1993, 100, 97–102. [Google Scholar] [CrossRef]
- Sfriso, A.; Pavoni, B. Macroalgae and phytoplankton competition in the central Venice Lagoon. Environ. Tech. 1994, 15, 1–14. [Google Scholar] [CrossRef]
- Tang, D.; Kawamura, H.; Doan-Nhu, H.; Takahashi, W. Remote sensing oceanography of a harmful algal bloom (HAB) off the coast of Southeastern Vietnam. J. Geophys. Res. Oceans 2004, 109. [Google Scholar] [CrossRef]
- Tang, D.; Kawamura, H.; Sang Oh, I.; Baker, J. Satellite evidence of harmful algal blooms and related oceanographic features in the Bohai Sea during autumn 1998. Adv. Space Res. 2006, 37, 681–689. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, J.; Sun, L.; Jia, Y.; Zhao, W.; Wang, Z.; Meng, J. Satellite monitoring of massive green macroalgae bloom (GMB): Imaging ability comparison of multi-source data and drifting velocity estimation. Int. J. Remote Sens. 2012, 33, 5513–5527. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M. Green macroalgae blooms in the Yellow Sea during the spring and summer of 2008. J. Geophys. Res. Oceans 2009, 114. [Google Scholar] [CrossRef]
- Edelvang, K.; Kaas, H.; Erichsen, A.C.; Alvarez-Berastegui, D.; Bundgaard, K.; Jugensen, P.V. Numerical modelling of phytoplankton biomass in coastal waters. J. Mar. Syst. 2005, 57, 13–19. [Google Scholar] [CrossRef]
- Ma, J.; Zhan, H.; Du, Y. Seasonal and interannual variability of surface CDOM in the South China Sea associated with El Niño. J. Mar. Syst. 2011, 85, 86–95. [Google Scholar]
- Liu, C.; Tang, D. Spatial and temporal variations in algal blooms in the coastal waters of the western South China Sea. J. Hydrol. Environ. Res. 2012, 6, 239–247. [Google Scholar] [CrossRef]
- Miller, R.L.; López, R.; Mulligan, R.P.; Reed, R.E.; Liu, C.C.; Buonassissi, C.J.; Brown, M.M. Examining material transport in dynamic coastal environments: An integrated approach using field data, remote sensing and numerical modeling. In Remote Sensing and Modeling: Advances in Coastal and Marine Resources Coastal Research Library; Finkl, C.W., Makowski, C., Eds.; Springe: New York, NY, USA, 2014; pp. 333–364. [Google Scholar]
- Dien, T.V.; Tang, D.; Kawamura, H. Validation SeaWiFS-Derived ocean color data and using for study distribution chlorophyll-a concentration in the Vietnam waters. In Proceedings of the 2003 Regional Conference on Digital GMS, Bangkok, Thailand, 26–28 February 2003; pp. 75–87.
- Nagamani, P.V.; Hussain, M.I.; Choudhury, S.B.; Panda, C.R.; Sanghamitra, P.; Kar, R.N.; Das, A.; Ramana, I.V.; Rao, K.H. Validation of chlorophyll-a algorithms in the coastal waters of Bay of Bengal initial validation results from OCM-2. J. Indian Soc. Remote Sens. 2013, 41, 117–125. [Google Scholar] [CrossRef]
- Kahru, M.; Kudela, R.M.; Anderson, C.R.; Manzano-Sarabia, M.; Mitchell, B.G. Evaluation of satellite retrievals of ocean Chlorophyll-a in the California Current. Remote Sens. 2014, 6, 8524–8540. [Google Scholar] [CrossRef]
- Li, G.; Gao, P.; Wang, F.; Liang, Q. Estimation of ocean primary productivity and its spatio-temporal variation mechanism for East China Sea based on VGPM model. J. Geogr. Sci. 2004, 14, 32–40. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M. Satellite views of the Bohai Sea, Yellow Sea, and East China Sea. Prog. Oceanogr. 2012, 104, 30–45. [Google Scholar] [CrossRef]
- Xing, Q.; Loisel, H.; Schmitt, F.G.; Dessailly, D.; Hao, Y.; Han, Q.; Shi, P. Fluctuations of satellite-derived chlorophyll concentrations and optical indices at the Southern Yellow Sea. Aquat. Ecosyst. Health Manag. 2012, 15, 168–175. [Google Scholar]
- Xia, B.; Ma, S.; Cai, Y.; Chen, B.; Chen, J.; Song, Y.; Mao, Y. Distribution of temperature, salinity, dissolved oxygen, nutrients and their relationships with green tide in Enteromorpha prolifera outbreak area of the Yellow Sea. Progr. Fish. Sci. 2009, 30, 94–101. (In Chinese) [Google Scholar]
- Liu, F.; Pang, S.; Chopin, T.; Gao, S.; Shan, T.; Zhao, X.; Li, J. Understanding the recurrent large-scale green tide in the Yellow Sea: Temporal and spatial correlations between multiple geographical, aquacultural and biological factors. Mar. Environ. Res. 2013, 83, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ning, J.; Shang, D.; Zhai, Y. Analysis of inorganic elements of Enteramorpha prolifera from Qingdao coasts in 2008. J. Biol. 2010, 27, 92–93. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Q.; Hu, C.; Tang, D.; Tian, L.; Tang, S.; Wang, X.H.; Lou, M.; Gao, X. World’s Largest Macroalgal Blooms Altered Phytoplankton Biomass in Summer in the Yellow Sea: Satellite Observations. Remote Sens. 2015, 7, 12297-12313. https://0-doi-org.brum.beds.ac.uk/10.3390/rs70912297
Xing Q, Hu C, Tang D, Tian L, Tang S, Wang XH, Lou M, Gao X. World’s Largest Macroalgal Blooms Altered Phytoplankton Biomass in Summer in the Yellow Sea: Satellite Observations. Remote Sensing. 2015; 7(9):12297-12313. https://0-doi-org.brum.beds.ac.uk/10.3390/rs70912297
Chicago/Turabian StyleXing, Qianguo, Chuanmin Hu, Danling Tang, Liqiao Tian, Shilin Tang, Xiao Hua Wang, Mingjing Lou, and Xuelu Gao. 2015. "World’s Largest Macroalgal Blooms Altered Phytoplankton Biomass in Summer in the Yellow Sea: Satellite Observations" Remote Sensing 7, no. 9: 12297-12313. https://0-doi-org.brum.beds.ac.uk/10.3390/rs70912297