Analysis and Mapping of the Spectral Characteristics of Fractional Green Cover in Saline Wetlands (NE Spain) Using Field and Remote Sensing Data
Abstract
:1. Introduction
2. Study Area
3. Material and Methods
3.1. Sampling Sites and Instruments
3.2. Ground Photography and Auxiliary Field Data
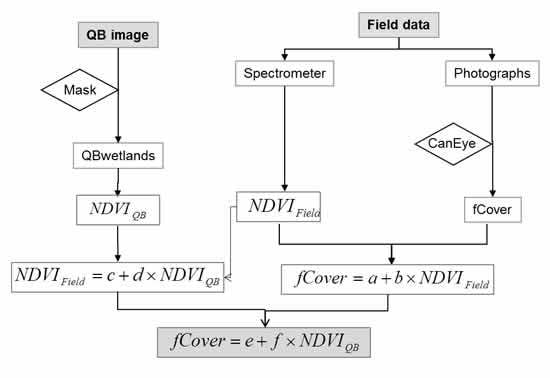
3.3. Satellite Images and Data Processing
4. Results
4.1. Spectral Characteristics of Soil and Vegetation
4.2. NDVI and Green Cover Fraction
4.3. NDVI Derived from Quickbird, ASTER, and Landsat
4.4. Maps of Vegetation
5. Discussion
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keramitsoglou, I.; Kontoes, C.; Sifakis, N.; Mitchley, J.; Xofis, P. Kernel based re-classification of earth observation data for fine scale habitat mapping. J. Nat. Conserv. 2005, 13, 91–99. [Google Scholar] [CrossRef]
- Bock, M.; Xofis, P.; Rossner, G.; Wissen, M.; Mitchley, J. Object oriented methods for habitat mapping in multiple scales: Case studies from Northern Germany and North Downs, GB. J. Nat. Conserv. 2005, 13, 75–89. [Google Scholar] [CrossRef]
- Boyd, D.S.; Sánchez-Hernández, C.; Foody, G.M. Mapping a specific class for priority habitats monitoring from satellite sensor data. Int. J. Remote Sens. 2006, 27, 2631–2644. [Google Scholar] [CrossRef]
- Kobler, A.; Dzeroski, S.; Keramitsoglou, L. Habitat mapping using machine learning-extended kernel-based reclassification of an Ikonos satellite image. Ecol. Model. 2006, 191, 83–95. [Google Scholar] [CrossRef]
- Nagendraa, H.; Lucas, R.; Honradoc, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Wang, L.; Sousa, W.; Gong, P.; Biging, G. Comparison of IKONOS and Quickbird images for mapping mangrove species on the Caribbean coast of Panama. Remote Sens. Environ. 2004, 91, 432–440. [Google Scholar] [CrossRef]
- Xiao, J.; Moody, A. A comparison of methods for estimating fractional green vegetation cover within a desert-to-upland transition zone in central New Mexico, USA. Remote Sens. Environ. 2005, 98, 237–250. [Google Scholar] [CrossRef]
- Wang, C.; Menenti, M.; Stoll, M.P.; Belluco, E.; Marani, M. Mapping mixed vegetation communities in salt marshes using airborne spectral data. Remote Sens. Environ. 2007, 107, 559–570. [Google Scholar] [CrossRef]
- Barati, S.; Raiegani, B.; Saati, M.; Sharifi, A.; Nasri, M. Comparison the accuracies of different spectral indices for estimation of vegetation cover fraction sparse vegetated areas. Egypt. J. Remote Sens. Space Sci. 2011, 14, 49–56. [Google Scholar] [CrossRef]
- Spanhove, T.; Borre, J.V.; Delalieux, S.; Haest, B.; Paelinckx, D. Can remote sensing estimate fine-scale quality indicators of natural habitats? Ecol. Indic. 2012, 18, 403–412. [Google Scholar] [CrossRef]
- Newman, M.E.; McLaren, K.P.; Wilson, B.S. Assessing deforestation and fragmentation in a tropical moist forest over 68 years; the impact of roads and legal protection in the Cockpit Country, Jamaica. For. Ecol. Manag. 2014, 315, 138–152. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.H.; Gong, H.L.; Li, X.J. Object-based urban tree species classification using Bi-Temporal Worldview-2 and Worldview-3 images. Remote Sens. 2015, 7, 16917–16937. [Google Scholar] [CrossRef]
- Rapinel, S.; Clement, B.; Magnanon, S.; Sellin, V.; Hubert-Moy, L. Identification and mapping of natural vegetation on a coastal site using a Worldview-2 satellite image. J. Environ. Manag. 2014, 114, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, T.; Freire, S. Testing the contribution of Worldview-2 improved spectral resolution for extracting vegetation cover in urban environments. Can. J. Remote Sens. 2015, 41, 501–514. [Google Scholar] [CrossRef]
- Smith, M.O.; Ustin, S.L.; Adams, J.B.; Gillespie, A.R. Vegetation in deserts: I. A regional measure of abundance from multispectral images. Remote Sens. Environ. 1990, 31, 1–26. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, J.L.; Wang, X.Q.; Gu, Z.H. The spatial distribution patterns of biological soil crusts in the Gurbantunggut Desert, Northern Xinjiang, China. J. Arid Environ. 2007, 68, 599–610. [Google Scholar] [CrossRef]
- Elmore, A.J.; Mustard, J.F.; Manning, S.J.; Lobell, D.B. Quantifying vegetation change in semi-arid environments: Precision and accuracy of spectral mixture analysis and the Normalized Difference Vegetation Index. Remote Sens. Environ. 2000, 73, 86–102. [Google Scholar]
- Camacho-De Coca, F.; García-Haro, F.; Gilabert, M.A.; Meliá, J. Vegetation cover seasonal changes assessment from TM imagery in a semi-arid landscape. Int. J. Remote Sens. 2004, 25, 3451–3476. [Google Scholar] [CrossRef]
- Schmid, T.; Koch, M.; Gumuzzio, J. Multisensor approach to determine changes of wetland characteristics in semi-arid environments (Central Spain). IEEE Trans. Geosci. Remote Sens. 2005, 43, 2516–2525. [Google Scholar] [CrossRef]
- Adamo, S.B.; Crews-Meyer, K.A. Aridity and desertification: Exploring environmental hazards in Jachal, Argentina. Appl. Geogr. 2006, 26, 61–85. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nakajlma, Y.; Kajiwara, K.; Tsuchiya, K. Extraction of vegetation cover in an arid area based on satellite data. Adv. Space Res. 1997, 19, 1375–1378. [Google Scholar] [CrossRef]
- Guerschman, J.P.; Hill, M.J.; Renzullo, L.J.; Barrett, D.J.; Marks, A.S.; Botha, E.J. Estimating fractional cover of photosynthetic vegetation, non-photosynthetic vegetation and bare soil in the Australian tropical savanna region upscaling the EO-1 Hyperion and MODIS sensors. Remote Sens. Environ. 2009, 113, 928–945. [Google Scholar] [CrossRef]
- Laliberte, A.; Fredrickson, E.; Rango, A. Combining decision trees with hierarchical object-oriented image analysis for mapping arid rangelands. Photogramm. Eng. Remote Sens. 2007, 73, 197–207. [Google Scholar] [CrossRef]
- Chuvieco, E. Teledetección Ambiental. La Observación de la Tierra Desde el Espacio; Ariel Ciencia: Barcelona, Spain, 2002. [Google Scholar]
- Duchemin, B.; Hadria, R.; Erraki, S.; Boulet, G.; Maisongrande, P.; Chehbouni, A.; Escadafal, R.; Ezzahar, J.; Hoedjes, J.C.B.; Kharrou, M.H.; et al. Monitoring wheat phenology and irrigation in Central Morocco: On the use of relationships between evapotranspiration, crops coefficients, leaf area index and remotely-sensed vegetation indices. Agric. Water Manag. 2006, 79, 1–27. [Google Scholar] [CrossRef]
- Jonckheere, I.; Nackaerts, K.; Muys, B.; Coppin, P. Assessment of automatic gap fraction estimation of forests from digital hemispherical photography. Agric. For. Meteorol. 2005, 132, 96–114. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Rango, A.; Havstad, K.M.; Paris, J.F.; Beck, R.F.; McNeely, R.; González, A.L. Object-oriented image analysis for mapping shrub encroachment from 1937–2003 in southern New Mexico. Remote Sens. Environ. 2004, 93, 198–210. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Cutler, M.E.J.; Lewis, H. Mapping a sub-pixel proportional land cover with AVHRR imagery. Int. J. Remote Sens. 1997, 18, 917–935. [Google Scholar] [CrossRef]
- DeFries, R.; Hansen, M.; Steininger, M.; Dubyah, R.; Sohlberg, R.; Townshend, J. Subpixel forest cover in Central Africa from multisensor, multitemporal data. Remote Sens. Environ. 1997, 60, 228–246. [Google Scholar] [CrossRef]
- McGwire, K.; Minor, T.; Fenstermaker, L. Hyperspectral mixture modeling for quantifying sparse vegetation cover in arid environments. Remote Sens. Environ. 2000, 72, 360–374. [Google Scholar] [CrossRef]
- Ju, J.; Kolaczyk, E.D.; Gopal, S. Gaussian mixture discriminant analysis and sub-pixel land cover characterization in remote sensing. Remote Sens. Environ. 2003, 84, 550–560. [Google Scholar] [CrossRef]
- Shi, C.; Wang, L. Incorporating spatial information in spectral unmixing: A review. Remote Sens. Environ. 2014, 149, 70–87. [Google Scholar] [CrossRef]
- Halabisky, M.; Moskai, L.M.; Gillespie, A.; Hanam, M. Reconstructing semi-arid wetland surface water dynamics through spectral mixture analysis of a time series of Landsat satellite images (1984–2011). Remote Sens. Environ. 2016, 177, 171–183. [Google Scholar] [CrossRef]
- Baghzouz, M.; Devitt, D.A.; Fenstermaker, L.F.; Young, M.H. Monitoring vegetation phenological cycles in two different semi-arid environmental settings using a ground-based NDVI system: A potential approach to improve satellite data interpretation. Remote Sens. 2010, 2, 990–1013. [Google Scholar] [CrossRef]
- Huete, A.R.; Jackson, R.D.; Post, D.F. Spectral response of a plant canopy with different soil backgrounds. Remote Sens. Environ. 1985, 17, 37–53. [Google Scholar] [CrossRef]
- Huete, A.R.; Jackson, R.D. Suitability of spectral indices for evaluating vegetation characteristics on arid rangeland. Remote Sens. Environ. 1987, 23, 213–232. [Google Scholar] [CrossRef]
- Escadafal, R.; Huete, A.R. Improvement in remote sensing of low vegetation cover in arid regions by correcting vegetation indices for soil “noise”. C. R. Acad. Sci. 1991, 312, 1385–1391. [Google Scholar]
- Adams, J.B.; Smith, M.O.; Gillespie, A.R. Imaging spectroscopy: Interpretation based on spectral mixture analysis. In Remote Geochemical Analysis Elemental and Mineralogical Composition; Pieters, C.M., Englert, P.A.J., Eds.; Press Syndicate of University of Cambridge: Cambridge, UK, 1993; pp. 145–166. [Google Scholar]
- Todd, S.W.; Hoffer, R.M. Responses of spectral indices to variations in vegetation cover and soil background. Photogramm. Eng. Remote Sens. 1998, 64, 915–921. [Google Scholar]
- Montandon, L.M.; Small, E.E. The impact of soil reflectance on the quantification of the green vegetation fraction from NDVI. Remote Sens. Environ. 2008, 112, 1835–1845. [Google Scholar] [CrossRef]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef]
- Maas, S.J. Estimating cotton canopy ground cover from remotely sensed scene reflectance. Agron. J. 1998, 90, 384–388. [Google Scholar] [CrossRef]
- Weber, B.; Olehowski, C.; Knerr, T.; Hill, J.; Deutschewitz, K.; Wessels, D.C.J.; Eitel, B.; Büdel, B. A new approach for mapping of biological soil crusts in semidesert areas with hyperspectral imagery. Remote Sens. Environ. 2008, 211, 2187–2201. [Google Scholar] [CrossRef]
- Ustin, S.L.; Valko, P.G.; Kefauver, S.C.; Santos, M.J.; Zimpfer, J.F.; Smith, S.D. Remote sensing of biological soil crust under simulated climate change manipulations in the Mojave Desert. Remote Sens. Environ. 2009, 113, 317–328. [Google Scholar] [CrossRef]
- Rozenstein, O.; Karnieli, A. Identification and characterization of biological soil crusts in a sand dune desert environment using LWIR emittance spectroscopy. J. Arid Environ. 2015, 112, 75–86. [Google Scholar] [CrossRef]
- Herrero, J.; Castañeda, C. Temporal changes in soil salt-affection and the salinity profiles at four hypersaline wetlands in NE Spain. Catena 2015, 133, 145–156. [Google Scholar] [CrossRef]
- Castañeda, C.; Herrero, J. Assessing the degradation of saline wetlands in an arid agricultural region in Spain. Catena 2008, 72, 205–213. [Google Scholar] [CrossRef]
- Conesa, J.A.; Castañeda, C.; Pedrol, J. Las Saladas de Monegros y su Entorno. Hábitats y Paisaje Vegetal; Consejo de Protección de la Naturaleza de Aragón: Zaragoza, Spain, 2011. Available online: http://digital.csic.es/handle/10261/109666 (accessed on 11 July 2016).
- Herrero, J.; Snyder, R.L. Aridity and irrigation in Aragón, Spain. J. Arid Environ. 1997, 35, 55–547. [Google Scholar] [CrossRef]
- Faci, J.M.; Martínez-Cob, A. Cálculo de la Evapotranspiración de Referencia en Aragón; Diputación General de Aragón: Zaragoza, Spain, 1991. [Google Scholar]
- Castañeda, C.; Herrero, J.; Conesa, J.A. Distribution, morphology and habitats of saline wetlands: A case study from Monegros, Spain. Geol. Acta 2013, 11, 371–388. [Google Scholar]
- Domínguez-Beisiegel, M.; Castañeda, C. Revisión histórica y actualización del inventario de humedales salinos de Monegros Sur. Base para una propuesta RAMSAR. In Tecnologías de la Información Geográfica para el Desarrollo Territorial; Hernández, L., Parreño, J.M., Eds.; Servicio de Publicaciones y Difusión Científica de la ULPGC: Las Palmas, Spain, 2008; pp. 564–575. [Google Scholar]
- Neuendorf, K.K.E.; Mehl, J.P., Jr.; Jackson, J.A. Glossary of Geology, 5th ed. (revised); American Geosciences Institute: Alexandria, VA, USA, 2011. [Google Scholar]
- Mees, F.; Castañeda, C.; Herrero, J.; van Ranst, E. The nature and significance of variations in gypsum crystal morphology in dry lake basins. J. Sediment. Res. 2012, 82, 37–52. [Google Scholar] [CrossRef]
- European Commission, DG-ENV. Interpretation Manual of European Union Habitats, Version EUR 28. 2013. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 25 April 2016).
- De Galán Mera, A.; Hagen, M.A.; Vicente Orellana, J.A. Aerophyte, a new life form in Raunkiaer’s classification? J. Veg. Sci. 2009, 10, 65–68. [Google Scholar] [CrossRef]
- Domínguez-Beisiegel, M.; Herrero, J.; Castañeda, C. Saline wetlands’ fate in inland deserts: An example of eighty years decline from Monegros, Spain. Land Degrad. Dev. 2013, 24, 250–265. [Google Scholar]
- Weiss, M.; Baret, F.; Smith, G.J.; Jonckheere, I.; Coppin, P. Review of methods for in situ leaf area index (LAI) determination. Part II. Estimation of LAI, errors and sampling. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Kallel, A.; Le Hegarat-Mascle, S.; Ottle, C.; Hubert-Moy, L. Determination of vegetation cover fraction by inversion of a four-parameter model based on isoline parametrization. Remote Sens. Environ. 2007, 111, 553–566. [Google Scholar] [CrossRef]
- Herrero, J.; Artieda, O.; Hudnall, W.H. Gypsum, a Tricky Material. Soil Sci. Soc. Am. J. 2009, 73, 1757–1763. [Google Scholar] [CrossRef]
- Domínguez-Beisiegel, M.; Castañeda, C.; Herrero, J. Two Microenvironments at the soil surface of saline wetlands in Monegros, Spain. Soil Sci. Soc. Am. J. 2013, 77, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Kubiena, W.L. The Soils of Europe. Illustrated Diagnosis and Sistematics; CSIC, Madrid & Thomas Murby and Co.: London, UK, 1953. [Google Scholar]
- Baret, F.; Jacquemond, S.; Hanocq, J.F. About the soil line concept in remote sensing. Adv. Space Res. 1993, 13, 281–284. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. CAN-EYE User Manual. CAN-EYE V6.1, EMMAH Laboratory (Mediterranean Environment and Agro-Hydro System Modelisation) in the French National Institute of Agricultural Research (INRA). Available online: https://www6.paca.inra.fr/can-eye/Download (accessed on 11 July 2016).
- Escadafal, R.; Bacha, S. Strategy for the dynamic study of desertification. In Monitoring Soils in the Environment with Remote Sensing and GIS; ORSTOM Editions: Paris, France, 1996; pp. 19–34. [Google Scholar]
- Salisbury, J.W.; D’Aria, D. Emissivity of terrestrial materials in the 8–14 µm atmospheric window. Remote Sens. Environ. 1992, 42, 83–106. [Google Scholar] [CrossRef]
- Tueller, P.T. Remote sensing science application in arid environment. Remote Sens. Environ. 1987, 23, 143–154. [Google Scholar] [CrossRef]










| Image Data | Satellite | |||||
|---|---|---|---|---|---|---|
| Quickbird | ASTER | Landsat 5TM | ||||
| Acquisition date | 2007 | 2008 | 2007 | 2007 | ||
| 11 July | 29 July | 28 August | 11 July | 11 July | ||
| Acquisition time | 11:15:50 | 11:15:52 | 11:15:55 | 11:19:11 | 10:56:29 | 10:25 |
| Part of the study area imaged | North | South-west | East | Center | Whole area | Whole area |
| Nadir angle (degrees) | 12.9 | 13.4 | 2.3 | 24.5 | 2.8 | nd |
| Format delivery | GeoTiff | HDF-EOS | CEOS | |||
| Quantization levels (data type) | 16 bits | 8 bits | ||||
| Correction Level | Standard2a | Sensor radiance 1B | System corrected 1A | |||
| Spectral range used | VIS, NIR | VIS, NIR, SWIR/MIR | ||||
| Spatial resolution (m) | 2.44–2.88 (VIS and NIR) | 15 (NIR) 30 (SWIR) | 30 | |||
| Area of original scene (km2) | 91.59 | 138.9 | 28.8 | 68.9 | 60 × 60 | 180 × 180 |
| Soil Color | Soil | Green | Yellow-Green | Woody | Mixed |
|---|---|---|---|---|---|
| Dark soils (chroma 4 or less) | 32.1 | 13.5 | 16.5 | 10.5 | 27.3 |
| Light soils (chroma 6–7) | 24.7 | 11.3 | 13.6 | 18.1 | 29.3 |
| Vegetation Classes (NDVIField > 0) | Bare Soil and Water Classes (NDVIField < 0) | ||
|---|---|---|---|
| fCover Threshold | Vegetation Class | NDVIField Threshold | Moisture Class |
| 0 ≤ 10 | Rare | 0–−0.05 | Dry |
| 10 ≤ 20 | Very sparse | −0.05–−0.3 | Moist (≤20%) |
| 20 ≤ 30 | Sparse | −0.3–−0.05 | Saturated (>20%) |
| 30 ≤ 40 | Medium sparse | <−0.05 | Water |
| 40 ≤ 50 | Low dense | ||
| >50 | Dense | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Beisiegel, M.; Castañeda, C.; Mougenot, B.; Herrero, J. Analysis and Mapping of the Spectral Characteristics of Fractional Green Cover in Saline Wetlands (NE Spain) Using Field and Remote Sensing Data. Remote Sens. 2016, 8, 590. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8070590
Domínguez-Beisiegel M, Castañeda C, Mougenot B, Herrero J. Analysis and Mapping of the Spectral Characteristics of Fractional Green Cover in Saline Wetlands (NE Spain) Using Field and Remote Sensing Data. Remote Sensing. 2016; 8(7):590. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8070590
Chicago/Turabian StyleDomínguez-Beisiegel, Manuela, Carmen Castañeda, Bernard Mougenot, and Juan Herrero. 2016. "Analysis and Mapping of the Spectral Characteristics of Fractional Green Cover in Saline Wetlands (NE Spain) Using Field and Remote Sensing Data" Remote Sensing 8, no. 7: 590. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8070590