4.1. Non-Destructive Estimation of Spring Maize Nitrogen Nutrition Index
In this study, five approaches were evaluated for estimating spring maize NNI. The first two approaches can be classified as mechanistic methods and the rest three approaches can be classified as semi-empirical methods, according to Chen [
16].
The first mechanistic method to NNI estimation is NNI–PNC–NDVI (or RVI), which requires the estimation of AGB and PNC. The results of this study indicated that AGB could be estimated well using GreenSeeker sensor across V5 to V10 (
R2 = 0.87–0.89). However, NDVI became saturated at the V10 stage when AGB was greater than 3 t·ha
−1. This should not limit its practical applications because most N management strategies recommend side-dressing N fertilizers to be applied before the V10 stage. High clearance fertilizer application machines will be needed at later growth stages and yield losses may occur. Thomason et al. [
22] found a liner plus plateau relationship between GreenSeeker NDVI and maize biomass across growth stages from V5 to V9 growth stages and NDVI reached a plateau at 0.82. Other researchers also reported the saturation effects of NDVI for winter wheat [
5,
32,
35] and rice [
19,
36]. The saturation effect of NDVI was mainly due to the canopy closure, the differences in penetration into the canopy between visible light (R) and NIR, and the normalization effect embedded in the calculation formula of this index [
37]. The saturation effect could be reduced using VIs with wavebands of similar penetration into crop canopy or using ratio indices [
37], as indicated by the relationship between AGB and RVI up to about 6 t·ha
−1 (
Figure 7b). Previous research indicated that RVI did not become saturated even after AGB reached 6 t·ha
−1, but the relationships became more scattered [
5,
19]. Nguy-Robertson et al. [
38] also reported that the NDVI index was most sensitive to LAI below 2 m
2·m
−2, while ratio indices (e.g., RVI) were most sensitive to LAI above this threshold. They suggested that NDVI and RVI should be combined for estimating LAI for improved sensitivity [
38]. This possibility should be evaluated for estimating maize AGB.
Maize PNC was slightly better estimated using the GreenSeeker sensor across growth stages (
R2 = 0.46) than the results previously reported for rice (
R2 < 0.40) [
19] and winter wheat (
R2 = 0.08–0.41) [
5]. The results for specific growth stages were much worse. Such results confirmed the difficulty to reliably estimate PNC at early crop growth stages before canopy closure, mainly because AGB increases faster than the plant uptake of N during this period and dominates canopy reflectance [
5,
19,
39]. The influence of the soil background may not be a problem, because the active light intensity decreases with measuring distance and follows the inverse square law, and the GreenSeeker sensing depth was confined to the upper canopy layer [
40]. It has been found that the canopy chlorophyll content index (CCCI) based on the theory of two-dimensional planar domain was highly related to summer maize PNC at the V6–V7 growth stages (
R2 = 0.65–0.68) [
41]. This integrated index uses NDVI as a surrogate for ground cover to separate soil signal from plant signal and normalized difference red edge (NDRE) as a measure of canopy N status, thus allowing a relative measure of plant N status while minimizing the influence of ground cover [
42]. Li et al. [
41] used passive hyperspectral reflectance data to simulate the bands of active sensor Crop Circle ACS 430, and calculated CCCI. However, it should be noted that the passive canopy sensors can detect the N status of the entire maize foliage, while active optical sensors can only detect the upper canopy layer [
40]. Therefore, the performance of AOS-based CCCI for estimating maize PNC has yet to be confirmed.
The estimation of PNC can also be improved using hyperspectral remote sensing. Chen et al. [
43] proposed a new spectral index, Double-peak Canopy Nitrogen Index (DCNI), which worked well for estimating maize PNC (
R2 = 0.72). Cilia et al. [
9] used aerial hyperspectral remote sensing and found an integrated index, Modified Chlorophyll Absorption Ratio Index/Modified Triangular Vegetation Index 2 (MCARI/MTVI2), performed well for estimating maize PNC (
R2 = 0.59), and they used this mechanistic method to estimate maize NNI (
R2 = 0.70). Chen [
16] also found that when using hyperspectral canopy sensing, this mechanistic method worked very well for winter wheat NNI estimation (
R2 = 0.82–0.94 for validation results).
The second approach is NNI–PNU–NDVI (or RVI), which is also a mechanistic method requiring the estimation of AGB and PNU. Maize PNU was well estimated across growth stages (
R2 = 0.83–0.84), but GreenSeeker NDVI became saturated when PNU reached about 80 kg·ha
−1, which was lower than the values reported for rice (about 100 kg·ha
−1) [
19] and winter wheat (131–135 kg·ha
−1) [
5,
32]. Perhaps this threshold value was affected by the plant height. Maize plants are much taller than rice and winter wheat and the GreenSeeker sensor can only sense the upper canopy layer [
40]. Freeman et al. [
44] found a new index, NDVI × Plant height, to be an excellent predictor of maize PNU and performed well in later growth stages (V11-R1 growth stages). This should be investigated to overcome the saturation problem of NDVI. This mechanistic method has also been taken for rice NNI estimation [
10].
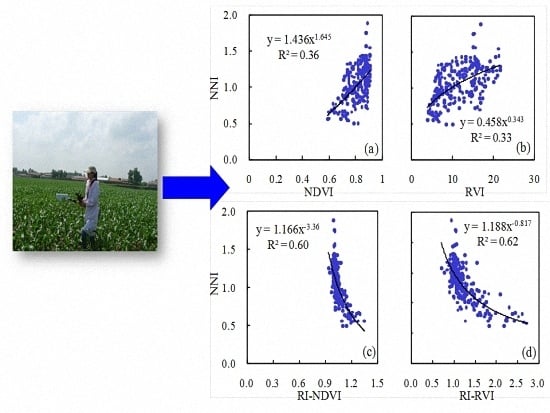
The third approach is NNI–NDVI (or RVI), which is a semi-empirical method. The results of this study indicated that maize NNI was better estimated using the GreenSeeker sensor at specific growth stages than across growth stages. This agreed with previous results reported for other crops [
5,
16,
19]. For rice, GreenSeeker VIs were moderately related to NNI at stem elongation (
R2 = 0.25–0.34) and heading (
R2 = 0.30–0.31) stages, but only weekly across growth stages (
R2 = 0.07–0.11) [
19]. For winter wheat, the
R2 values for the relationships between GreenSeeker VIs and NNI were 0.52–0.54, 0.55–0.64 and 0.44–0.47 at Feekes growth stages 4–7, 8–10, and across growth stages, respectively [
5]. This may be due to the fact that NNI is a relative value not significantly influenced by growth stages while GreenSeeker NDVI and RVI are significantly related to AGB, which increases very fast at the vegetative growth stages. The sensitivity to the influence of phenology (or growth stages) will limit the practical application of this semi-empirical method for NNI estimation [
16].
The fourth approach, NNI–RI–NDVI (or RVI), is also a semi-empirical method, but it performed consistently well either across growth stages or at specific growth stages (
R2 = 0.56–0.68). The RI is a relative value normalized by the well-fertilized reference plots, which have been commonly used to reduce the influence of other confounding factors on sensor-based N status diagnosis and eliminate the need to develop site-specific calibrations [
45]. A similar approach using relative CM readings was used to improve estimation of maize NNI in Canada [
13]. This approach effectively overcame the influence of phenology on the performance of the first semi-empirical approach for estimating NNI.
4.2. In-Season Non-Destructive Diagnosis of Maize N Status
After maize NNI is non-destructively predicted, it is necessary to determine the threshold values. Based on the relationship between relative yield and NNI (
Figure 4), the NNI thresholds of <0.95, 0.95–1.05, and >1.05 were proposed to indicate deficient, optimal, and surplus N status. The lower threshold value of 0.95 was chosen with the consideration to be on the conservative side and grain yield would not be reduced significantly. These threshold values were the same as those proposed by Huang et al. [
10] for rice and were more suitable for this study than the thresholds proposed by Cilia et al. [
9] for maize. If the lower threshold value of 0.9 as proposed by Cilia et al. [
9] was adopted, the relative yield would be 0.84, which was 16% yield reduction compared with 100% relative yield or it would be 0.88 compared with 95.5% relative yield (when NNI was >0.96), which was 12% yield reduction. This would not be acceptable to farmers. Such threshold values may need to be adjusted for different regions.
Based on the predicted NNI using the four approaches discussed previously and the NNI threshold values, maize N status can be diagnosed. The results of this study indicated that the NNI–RI–NDVI approach performed the best at the V7–V8 stage, with accuracy rate of 76%. The fifth approach used the relationship between NNI and RI–NDVI (or RI–RVI), and determined the corresponding threshold values of RI–NDVI (or RI–RVI) when NNI was 0.95 and 1.05. Such RI–NDVI (or RI–RVI) threshold values can be used directly for N status diagnosis without the need to predict NNI. The results of this study indicated that the RI–NDVI approach performed even better than NNI–RI–NDVI at the V7–V8 stage, with the accuracy rate of 81%. The RI was proposed by Johnson and Raun [
46] to predict the crop response to additional N fertilizer application and previous research results indicated that RI–NDVI and grain yield at harvest were significantly correlated [
47,
48]. At the V9–V10 stages, all the five approaches performed similarly. These results indicated that the RI–NDVI approach was a reliable and simple approach that can be easily adopted by farmers. More studies are needed to determine if these threshold values are applicable across diverse on-farm conditions in Northeast China.
4.3. Implications for Precision Nitrogen Management of Spring Maize
The N fertilizer application rates by farmers varied significantly from farmer to farmer with the proportion of under-application, optimum rate and over-application being about 1/3 each, respectively [
4]. The first step to improve the farmer’s N management is to recommend a regional optimum N rate (RONR). A moderate amount of the RONR can be applied as basal fertilizer, and AOS can be used to diagnose the crop N status before side-dressing N application and the actual side-dressing N rate can be adjusted based on the N status diagnosis results [
18]. Although the critical N dilution curve for spring maize in Northeast China has been established [
24], it is still not practical to use NNI for in-season site-specific N status diagnosis.
The results of this study indicated that the GreenSeeker AOS could be used to estimate spring maize NNI non-destructively, especially using RI–NDVI or RI–RVI. The diagnosis accuracy rate was acceptable at V7–V8 and V9–V10 growth stages (71%–81%). The V7–V8 stage was the optimum stage recommended for side-dressing N application. Sometimes it can be delayed to V9–V10 stage due to bad weather or labor shortage. Theoretically, the normalization of GreenSeeker VIs using the well-fertilized reference plots or strips in the form of RI will eliminate the need for site-specific calibrations of the established relationships or models and this would greatly facilitate the application of NNI for in-season site-specific N status diagnosis. Based on the results of this study, it is recommended that RI–NDVI should be used to diagnose maize N status directly at V7–V8 growth stages while either RI–NDVI or RI–RVI can be used at the V9–V10 growth stages. This makes it more practical and convenient to use the sensors for in-season N status diagnosis without the need to estimate NNI after the threshold values are established. Further studies are needed to evaluate these threshold values and determine how many N rich strips should be used, how they should be arranged, and how to account for the variability within the reference strip(s) [
49], especially under small scale Chinese farming systems.
After maize N status is determined, the side-dressing N rate of the RONR can be increased or decreased by a fixed amount if the crop N status is N deficient or N surplus. A more quantitative approach proposed by Huang et al. [
10] is to use remote sensing technology to estimate biomass and PNU before side-dressing N application, and the difference between PNU and PNU
c can be calculated. The final side-dressing N application rate can be calculated as side-dressing N rate of RONR—(PNU–PNU
c). In addition to the NNI approach, the side-dressing N application rate can be determined using the N fertilizer optimization algorithm [
50] or empirical relationships between relative sensor readings and side-dressing N rates [
51].
Cilia et al. [
9] evaluated an aerial hyperspectral remote sensing approach to estimate maize NNI at V10 growth stage. They suggested to use remote sensing technology to estimate maize biomass (
R2 = 0.80) and PNC (
R2 = 0.59), NNI can be calculated using the estimated PNC and the calculated N
c. They found that the mean NNI estimated for each field parcel was significantly correlated with the NNI determined destructively (
R2 = 0.70). Our results indicated that similar results could be achieved with the GreenSeeker sensor. For large area applications of the proposed approaches, satellite or aerial remote sensing technologies may be more practical. However, images cannot be obtained under bad weather or cloudy conditions which can limit their on-farm applications. The active sensing systems don’t have such limitations. They have been installed on fertilizer application machines and on-the-go sensing and variable rate side-dressing N application have already been conducted [
51]. Such N sensing and fertilizer application systems need to be developed and evaluated in China. Future research also needs to investigate the potential of improving spring maize N status diagnosis using active canopy sensors with red edge band like Crop Circle ACS-470 or Crop Circle ACS-430 sensors (Holland Scientific, Lincoln, NE, USA) [
5,
41].
Although active sensors are advantageous since they are not influenced by the ambient light conditions, their disadvantages of reducing light intensity with measuring distance cannot be ignored, especially for tall row crops [
40,
52]. The measuring distance between the GreenSeeker sensor and the crop canopy should be maintained in the range of 0.7–1.4 m to collect stable sensor readings [
52]. The Crop Circle ACS-430 sensor has a unique feature that the reflectance data collected by this sensor are not affected by measuring distance in the range of 0.3–2.0 m. It also has a red edge band, in addition to red and NIR bands. A recent study indicated that the GreenSeeker VIs were not significantly related to rice yield or rice yield responsiveness to N fertilization at the heading stage, while red edge-based VIs obtained with Crop Circle ACS-470 sensor were still significantly related to these parameters (
R2 = 0.68–0.75) and effectively overcame the saturation problem of NDVI [
53]. More studies are needed to determine the potential of Crop Circle sensor to improve crop N status diagnosis and side-dressing N recommendation for maize.
One thing to be noted is that only two plant samples were collected from each plot in this study, due to the challenge to collect large number of maize plant samples with high frequencies. Care was taken to determine plant height and leaf age of five randomly selected plant samples in each plot and use this information as reference to select two representative plant samples for destructive sampling and analysis. Chen et al. [
54] also collected three maize plants in their research. Even so, future research should consider increasing sampling size to minimize errors.