Contrasting Responses of Planted and Natural Forests to Drought Intensity in Yunnan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Materials
2.2.1. MODIS Data
2.2.2. Forests’ Inventory Data
2.2.3. Meteorological Drought Index
2.3. Methods
2.3.1. Selecting the Optimal SPEI
2.3.2. Drought Intensity-Related Indicators
2.3.3. Comparisons between Planted and Natural Forests
3. Results
3.1. Optimal SPEI for the Planted and Natural Forests
3.2. Potential Impacts of Drought on Forests
3.3. Comparisons of the Drought Responses by Planted and Natural Forests
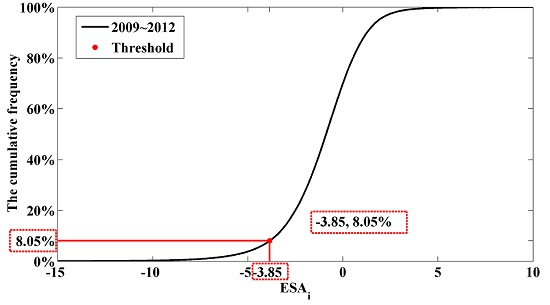
3.4. Responses of Planted and Natural Forests to Drought Intensity
3.5. Application of the ForestApproximate Threshold in Small Regions
4. Discussion
4.1. Ecological Significance of the Optimal SPEI
4.2. Differences between Planted and Natural Forests
4.3. Potential Effects of Different Spatial Scales
4.4. Limitations and Prospects
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Dai, A.; Trenberth, K.E.; Qian, T.T. A global dataset of palmer drought severity index for 1870–2002: Relationship with soil moisture and effects of surface warming. J. Hydrometeorol. 2004, 5, 1117–1130. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P. The interplay between climate change, forests, and disturbances. Sci. Total Environ. 2000, 262, 201–204. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q.; Dokken, D.J.; Ebi, K.L.; Mastrandrea, M.D.; Mach, K.J.; Plattner, G.K.; Allen, S.K.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change: Cambridge, UK, 2012; p. 582. [Google Scholar]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrio-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gegout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Pawson, S.M.; Brin, A.; Brockerhoff, E.G.; Lamb, D.; Payn, T.W.; Paquette, A.; Parrotta, J.A. Plantation forests, climate change and biodiversity. Biodivers. Conserv. 2013, 22, 1203–1227. [Google Scholar] [CrossRef]
- Klein, T.; Yakir, D.; Buchmann, N.; Gruenzweig, J.M. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014, 201, 712–716. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Fisher, R.A.; Xu, C.; Domec, J.C.; Holtta, T.; Mackay, D.S.; Sperry, J.S.; Boutz, A.; Dickman, L.; Gehres, N.; et al. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytol. 2013, 200, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, C.; Cox, P.M.; Lenton, T.M. Contrasting responses of a simple terrestrial ecosystem model to global change. Ecol. Model. 2000, 134, 41–58. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Meason, D.F.; Mason, W.L. Evaluating the deployment of alternative species in planted conifer forests as a means of adaptation to climate change-case studies in new zealand and scotland. Ann. For. Sci. 2014, 71, 239–253. [Google Scholar] [CrossRef]
- Bremer, L.L.; Farley, K.A. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Sun, G.; Fang, X.; Zha, T.; McNulty, S.; Chen, J.; Jin, Y.; Noormets, A. Response of ecosystem carbon fluxes to drought events in a poplar plantation in northern china. For. Ecol. Manag. 2013, 300, 33–42. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, H. Productivity as related to diversity and age in planted versus natural forests. Glob. Ecol. Biogeogr. 2014, 23, 1461–1471. [Google Scholar] [CrossRef]
- Sloan, S.; Sayer, J.A. Forest resources assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For. Ecol. Manag. 2015, 352, 134–145. [Google Scholar] [CrossRef]
- Fashing, P.J.; Nga, N.; Luteshi, P.; Opondo, W.; Cash, J.F.; Cords, M. Evaluating the suitability of planted forests for african forest monkeys: A case study from kakamega forest, kenya. Am. J. Primatol. 2012, 74, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.-C.; King, J.S.; Ward, E.; Christopher Oishi, A.; Palmroth, S.; Radecki, A.; Bell, D.M.; Miao, G.; Gavazzi, M.; Johnson, D.M.; et al. Conversion of natural forests to managed forest plantations decreases tree resistance to prolonged droughts. For. Ecol. Manag. 2015, 355, 58–71. [Google Scholar] [CrossRef]
- Broeckx, L.S.; Verlinden, M.S.; Berhongaray, G.; Zona, D.; Fichot, R.; Ceulemans, R. The effect of a dry spring on seasonal carbon allocation and vegetation dynamics in a poplar bioenergy plantation. GCB Bioenergy 2014, 6, 473–487. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, Á.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. Natural rear-edge pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.-Q.; Wu, J.; Zong, S.-X.; Yao, G.-L.; Li, Y.; Liu, Y.-M.; Zhang, Y.-R. Community structure and biodiversity in plantations and natural forests of seabuckthorn in southern Ningxia, China. For. Stud. China 2009, 11, 49–54. [Google Scholar]
- Fernández, M.E.; Gyenge, J.; Schlichter, T. Water flux and canopy conductance of natural versus planted forests in Patagonia, south America. Trees 2008, 23, 415–427. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Berninger, F.; Duan, B. Net primary production of chinese fir plantation ecosystems and its relationship to climate. Biogeosciences 2014, 11, 5595–5606. [Google Scholar] [CrossRef]
- Samanta, A.; Ganguly, S.; Hashimoto, H.; Devadiga, S.; Vermote, E.; Knyazikhin, Y.; Nemani, R.R.; Myneni, R.B. Amazon forests did not green-up during the 2005 drought. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, C.; Li, W.; Fang, X.; Zhang, T.; Zhu, Q.; Chen, H.; Zhao, P. Monitoring and estimating drought-induced impacts on forest structure, growth, function, and ecosystem services using remote-sensing data: Recent progress and future challenges. Environ. Rev. 2013, 21, 103–115. [Google Scholar] [CrossRef]
- Xiao, J. Satellite evidence for significant biophysical consequences of the “grain for green” program on the loess plateau in china. J. Geophys. Res. Biogeosci. 2014, 119, 2261–2275. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhang, Y.; Wan, C. Carbon sequestration potential of the stands under the grain for green program in yunnan province, china. For. Ecol. Manag. 2009, 258, 199–206. [Google Scholar] [CrossRef]
- State Forestry Administration of the People’s Republic of China. The Main Results at Eighth National Forest Resources Inventory (2009–2013). 2015. Available online: http://www.forestry.gov.cn/main/65/content-659670.html (accessed on 8 October 2014). [Google Scholar]
- Yang, J.; Gong, D.; Wang, W.; Hu, M.; Mao, R. Extreme drought event of 2009/2010 over Southwestern China. Meteorol. Atmos. Phys. 2012, 115, 173–184. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, H.; Liang, S.; Zhou, T.; He, B.; Tang, B.; Wu, D. Responses of natural vegetation to different stages of extreme drought during 2009–2010 in Southwestern China. Remote Sens. 2015, 7, 14039–14054. [Google Scholar] [CrossRef]
- Vincent, G.; de Foresta, H.; Mulia, R. Co-Occurring tree species show contrasting sensitivity to enso-related droughts in planted dipterocarp forests. For. Ecol. Manag. 2009, 258, 1316–1322. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Sturtevant, B.R. Modeling forest mortality caused by drought stress: Implications for climate change. Ecosystems 2013, 16, 60–74. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Silander, J.A., Jr. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proc. Natl. Acad. Sci. USA 2015, 112, 13585–13590. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the modis vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Maeda, E.E.; Heiskanen, J.; Aragao, L.E.O.C.; Rinne, J. Can MODIS EVI monitor ecosystem productivity in the amazon rainforest? Geophys. Res. Lett. 2014, 41, 7176–7183. [Google Scholar] [CrossRef]
- Huang, K.; Yi, C.; Wu, D.; Zhou, T.; Zhao, X.; Blanford, W.J.; Wei, S.; Wu, H.; Ling, D.; Li, Z. Tipping point of a conifer forest ecosystem under severe drought. Environ. Res. Lett. 2015, 10, 024011. [Google Scholar] [CrossRef]
- Department of Land Resources of Yunnan Province. Yunnan Land and Resources Yearbook in 2011; Dehong Minorities Press: Luxi, China, 2012. [Google Scholar]
- Vicente-Serrano, S.M.; Begueria, S.; Lopez-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Julio Camarero, J.; Begueria, S.; Trigo, R.; Lopez-Moreno, J.I.; Azorin-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhou, T.; Zhao, X.; Huang, K.; Gao, S.; Wu, H.; Luo, H. Assessments of drought impacts on vegetation in china with the optimal time scales of the climatic drought index. Int. J. Environ. Res. Public Health 2015, 12, 7615–7634. [Google Scholar] [CrossRef] [PubMed]
- Jacques, F.M.B.; Guo, S.-X.; Su, T.; Xing, Y.-W.; Huang, Y.-J.; Liu, Y.-S.; Ferguson, D.K.; Zhou, Z.-K. Quantitative reconstruction of the late miocene monsoon climates of southwest China: A case study of the lincang flora from yunnan province. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 304, 318–327. [Google Scholar] [CrossRef]
- Anderson, L.O.; Malhi, Y.; Aragao, L.E.O.C.; Ladle, R.; Arai, E.; Barbier, N.; Phillips, O. Remote sensing detection of droughts in Amazonian forest canopies. New Phytol. 2010, 187, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Holben, B.N. Characteristics of maximum-value composite images from temporal avhrr data. Int. J. Remote Sens. 1986, 7, 1417–1434. [Google Scholar] [CrossRef]
- The Land Processes Distributed Active Archive Center. Available online: http://reverb.echo.nasa.gov/reverb/#utf8=%E2%9C%93spatial_type=rectangle (accessed on 3 January 2015).
- Paulo, A.A.; Rosa, R.D.; Pereira, L.S. Climate trends and behaviour of drought indices based on precipitation and evapotranspiration in Portugal. Nat. Hazards Earth Syst. Sci. 2012, 12, 1481–1491. [Google Scholar] [CrossRef]
- Asefi-Najafabady, S.; Saatchi, S. Response of african humid tropical forests to recent rainfall anomalies. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wei, X.; Wu, Y.; Liu, S.; Huang, Y.; Yan, J.; Zhang, D.; Zhang, Q.; Liu, J.; Meng, Z.; et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Chang. Biol. 2011, 17, 3736–3746. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J. Changes in drought characteristics over china using the standardized precipitation evapotranspiration index. J. Clim. 2015, 28, 5430–5447. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhao, X.; Huang, K.; Wu, H.; Du, L. Diverse spatiotemporal responses in vegetation growth to droughts in China. Environ. Earth Sci. 2015, 75, 1–13. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-Lag effects of global vegetation responses to climate change. Glob. Chang. Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef] [PubMed]
- The SPEI Calculation Procedure. Available online: http://digital.csic.es/handle/10261/10002 (accessed on 20 January 2015).
- China Meteorological Administration and the National Meteorological Information Center. The China Meteorological Data Sharing Service System 2015. Available online: http://cdc.nmic.cn (accessed on 10 January 2015).
- Zeinivand, H. Comparison of interpolation methods for precipitation fields using the physically based and spatially distributed model of river runoff on the example of the Gharesou Basin, Iran. Russ. Meteorol. Hydrol. 2015, 40, 480–488. [Google Scholar] [CrossRef]
- Meyers, L.S.; Gamst, G.C.; Guarind, A.J. Performing Data Analysis Using IBM SPSS; John Wiley Sons, LLC: Hoboken, NJ, USA, 2013; pp. 174–190. [Google Scholar]
- Ma, X.; Huete, A.; Moran, S.; Ponce-Campos, G.; Eamus, D. Abrupt shifts in phenology and vegetation productivity under climate extremes. J. Geophys. Res. Biogeosci. 2015, 120, 2036–2052. [Google Scholar] [CrossRef]
- The Forestry Department of Yunnan Province. The Survey Methods of the Loss of Forest Resources by Drought Disaster in Yunnan Province; The Forestry Department of Yunnan Province: Kunming, China, 2011.
- The State Forestry Bureau. China Forestry Statistial Yearbook 2009–2012; China Forestry Publishing House: Beijing, China, 2010.
- The State Forestry Bureau. China Forestry Statistial Yearbook 2009–2012; China Forestry Publishing House: Beijing, China, 2011.
- The State Forestry Bureau. China Forestry Statistial Yearbook 2009–2012; China Forestry Publishing House: Beijing, China, 2012.
- The State Forestry Bureau. China Forestry Statistial Yearbook 2009–2012; China Forestry Publishing House: Beijing, China, 2013.
- Ho, R. Handbook of Univariate and Multivariate Data Analysis with IBM SPSS; Chapman and Hall, LLC: London, UK, 2014; pp. 525–527. [Google Scholar]
- Wang, W.; Zeng, W.; Chen, W.; Yang, Y.; Zeng, H. Effects of forest age on soil autotrophic and heterotrophic respiration differ between evergreen and deciduous forests. PLoS ONE 2013, 8, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, J.; Lei, T.; He, B.; Wu, Z.; Liu, M.; Mo, X.; Geng, G.; Li, X.; Zhou, H.; et al. Temporal-Spatial characteristics of severe drought events and their impact on agriculture on a global scale. Quat. Int. 2014, 349, 10–21. [Google Scholar] [CrossRef]
- Carlos Linares, J.; Julio Camarero, J.; Antonio Carreira, J. Competition modulates the adaptation capacity of forests to climatic stress: Insights from recent growth decline and death in relict stands of the mediterranean fir abies pinsapo. J. Ecol. 2010, 98, 592–603. [Google Scholar] [CrossRef]
- Vizzarri, M.; Tognetti, R.; Marchetti, M. Forest ecosystem services: Issues and challenges for biodiversity, conservation, and management in Italy. Forests 2015, 6, 1810–1838. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nystrom, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Beth, M.; Jim, G. Biodiversity and ecosystem functioning:synthesis and perspectives. Restor. Ecol. 2004, 12, 611–612. [Google Scholar]
- Linares, J.C.; Taïqui, L.; Sangüesa-Barreda, G.; Seco, J.I.; Camarero, J.J. Age-Related drought sensitivity of Atlas Cedar (cedrus atlantica) in the moroccan middle Atlas forests. Dendrochronologia 2013, 31, 88–96. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Luo, Y. Net aboveground biomass declines of four major forest types with forest ageing and climate change in Western Canada’s boreal forests. Glob. Chang. Biol. 2015, 21, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Kolb, T.E. Drought responses of conifers in ecotone forests of Northern Arizona: Tree ring growth and leaf sigma(13)c. Oecologia 2004, 140, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Barbara, J.B. Age-Related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar]
- Nepstad, D.C.; Tohver, I.M.; Ray, D.; Moutinho, P.; Cardinot, G. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 2007, 88, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Zhang, J.F.; Yang, D.J.; Geng, Y.F. Comparative Study of Plant Diversity between Betula alnoides plantation forestss and Adjacent Natural Forests. Taiwan J. For. Sci. 2011, 26, 323–339. [Google Scholar]
- Ayup, M.; Hao, X.; Chen, Y.; Li, W.; Su, R. Changes of xylem hydraulic efficiency and native embolism of tamarix ramosissima ledeb. Seedlings under different drought stress conditions and after rewatering. South Afr. J. Bot. 2012, 78, 75–82. [Google Scholar] [CrossRef]
- February, E.; Manders, P. Effects of water supply and soil type on growth, vessel diameter and vessel frequency in seedlings of three fynbos shrubs and two forest trees. South Afr. J. Bot. 1999, 65, 382–387. [Google Scholar] [CrossRef]
- De Keersmaecker, W.; Lhermitte, S.; Tits, L.; Honnay, O.; Somers, B.; Coppin, P. A model quantifying global vegetation resistance and resilience to short-term climate anomalies and their relationship with vegetation cover. Glob. Ecol. Biogeogr. 2015, 24, 539–548. [Google Scholar] [CrossRef]
- Seddon, A.W.R.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [PubMed]










| Groups | Elevation | Forest Type |
|---|---|---|
| Group 1 | 0–2000 m | Coniferous forest |
| Group 2 | 2000–4000 m | Coniferous forest |
| Group 3 | 4000–6000 m | Coniferous forest |
| Group 4 | 0–2000 m | Mixed forest |
| Group 5 | 2000–4000 m | Mixed forest |
| Drought Severity | SPEI |
|---|---|
| Extreme drought | SPEI < −2 |
| Severe drought | −2 < SPEI < −1.5 |
| Moderate drought | −1.5 < SPEI < −1 |
| Mild drought | −1 < SPEI < −0.5 |
| Normal | −0.5 < SPEI < 0.5 |
| Mild wet | 0.5 < SPEI < 1 |
| Moderate wet | 1 < SPEI < 1.5 |
| Severe wet | 1.5 < SPEI < 2 |
| Extreme wet | SPEI > 2 |
| The Abbreviation | Definition |
|---|---|
| ESA | EVI standard anomaly. |
| T1, T2, T3 | Thresholds of −2, −3, and −3.85. |
| PT1, PT2, PT3 | Yearly proportions of ESA less than the thresholds of −2, −3, and −3.85, respectively. |
| PC, PM, NC, NM | Planted coniferous forests, planted mixed forests, natural coniferous forests and natural mixed forests, respectively. |
| D, DC, DM | Difference between planted and natural forests in PT3, difference between planted coniferous forests and natural coniferous forests, and difference between planted mixed forests and natural mixed forests, respectively. |
| Group | N | ESAJun < T1 | ESAJun < T2 | ESAJun < T3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-n | N-p | N-e | P1 | N-n | N-p | N-e | P2 | N-n | N-p | N-e | P3 | ||
| Group 1 | 14 | 7 | 7 | 0 | 0.272 | 2 | 6 | 6 | 0.036 | 2 | 6 | 6 | 0.050 |
| Group 2 | 14 | 1 | 13 | 0 | 0.022 | 1 | 7 | 6 | 0.025 | 1 | 7 | 6 | 0.017 |
| Group 3 | 14 | 9 | 5 | 0 | 0.975 | 4 | 4 | 6 | 0.779 | 4 | 4 | 6 | 1.000 |
| Group 4 | 14 | 4 | 10 | 0 | 0.030 | 1 | 7 | 6 | 0.05 | 1 | 7 | 6 | 0.050 |
| Group 5 | 14 | 7 | 7 | 0 | 0.510 | 6 | 2 | 6 | 0.327 | 6 | 2 | 6 | 0.484 |
| Forests Group | N | N-n (Percentage, Sum of Rank) | N-p (Percentage, Sum of Rank) | N-e (Percentage) | p |
|---|---|---|---|---|---|
| Coniferous | 127 | 55 (43.31%, 3235.00) | 72 (56.69%, 4893.00) | 0 (0%) | 0.046 ** |
| Mixed | 70 | 25 (35.71%, 814.50) | 41 (58.57%, 1396.50) | 4 (5.72%) | 0.063 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Zhou, T.; Wu, H.; Zhao, X.; Wang, Q.; Gao, S.; Li, Z. Contrasting Responses of Planted and Natural Forests to Drought Intensity in Yunnan, China. Remote Sens. 2016, 8, 635. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8080635
Luo H, Zhou T, Wu H, Zhao X, Wang Q, Gao S, Li Z. Contrasting Responses of Planted and Natural Forests to Drought Intensity in Yunnan, China. Remote Sensing. 2016; 8(8):635. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8080635
Chicago/Turabian StyleLuo, Hui, Tao Zhou, Hao Wu, Xiang Zhao, Qianfeng Wang, Shan Gao, and Zheng Li. 2016. "Contrasting Responses of Planted and Natural Forests to Drought Intensity in Yunnan, China" Remote Sensing 8, no. 8: 635. https://0-doi-org.brum.beds.ac.uk/10.3390/rs8080635