Phenocams Bridge the Gap between Field and Satellite Observations in an Arid Grassland Ecosystem
Abstract
:1. Introduction
- (1)
- describe the phenological profiles of co-existing grass and shrub species using field data and phenocam imagery;
- (2)
- assess how phenological metrics such as start, end, and length of growing season vary between phenocam imagery and field observations;
- (3)
- examine the relationship between growing season estimates of canopy greenness for grass and shrub species using field methods and phenocam imagery; and
- (4)
- explore how species-level phenological trends contribute to landscape-level greenness derived from both phenocam imagery and MODIS NDVI.
2. Materials and Methods
2.1. Study Site
2.2. Phenology Observations
2.2.1. Field
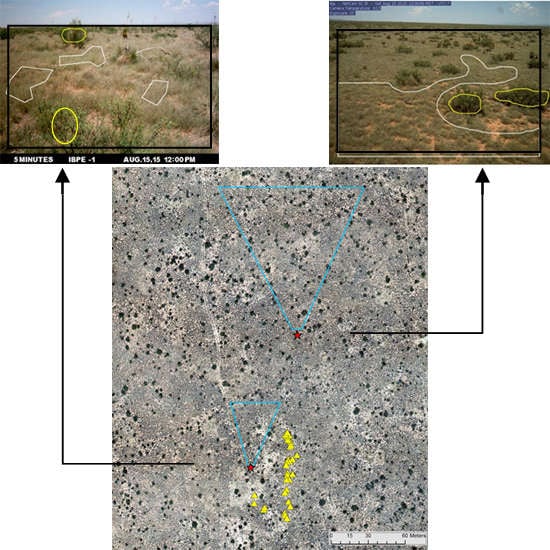
2.2.2. Phenocams
2.2.3. Phenocam Image Processing
2.3. Derivation of Seasonal Metrics
2.3.1. Field Phenophase Observations
2.3.2. Phenocams (Timesat)
2.3.3. MODIS NDVI (Timesat)
2.4. Data Analysis—Metric Comparisons
2.4.1. Seasonal Metrics (Field vs. PhenoCam, Objective 2)
2.4.2. Canopy Greenness (Field vs. Phenocam, Objective 3)
2.4.3. Landscape Greenness (Phenocam vs. MODIS, Objective 4)
3. Results
3.1. Phenological Profiles (Field and Phenocam, Objective 1)
3.2. Metric Comparisons
Seasonal Metrics (Field vs. Phenocam, Objective 2)
3.3. Landscape Greenness (Phenocam vs. MODIS)
4. Discussion
4.1. Species Phenology Profiles
4.2. Comparison of Phenology Metrics across Platforms
4.3. Management Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Species | Threshold (%) | Year | SOS | EOS | Duration (Days) |
|---|---|---|---|---|---|
| Black grama | 15 | 2012 | 228 | 290 | 62 |
| Black grama | 15 | 2013 | 208 | 289 | 81 |
| Black grama | 15 | 2014 | 191 | 307 | 116 |
| Black grama | 15 | 2015 | 184 | 346 | 162 |
| Black grama | 15 | 2016 | 222 | 324 | 102 |
| Mesquite | 15 | 2012 | 111 | 323 | 212 |
| Mesquite | 15 | 2013 | 116 | 334 | 218 |
| Mesquite | 15 | 2014 | 110 | 348 | 238 |
| Mesquite | 15 | 2015 | 129 | 330 | 201 |
| Mesquite | 15 | 2016 | 113 | 363 | 250 |
| Target | Threshold (%) | Year | SOS | EOS | Duration (Days) | Date of Peak GCC/NDVI |
|---|---|---|---|---|---|---|
| Land GCC | 10 | 2012 | 227 | 328 | 101 | 25 September 2012 |
| Land GCC | 10 | 2013 | 77 | 294 | 217 | 6 September 2013 |
| Land GCC | 10 | 2014 | 189 | 314 | 125 | 6 September 2014 |
| Land GCC | 10 | 2015 | 135 | 347 | 212 | 6 August 2015 |
| Land GCC | 10 | 2016 | 218 | NA | NA | 5 September 2016 |
| NDVI | 10 | 2012 | 75 | NA | NA | 27 July 2012 |
| NDVI | 10 | 2013 | 185 | 365 | 180 | 14 September 2013 |
| NDVI | 10 | 2014 | 156 | 365 | 209 | 29 August 2014 |
| NDVI | 10 | 2015 | 82 | 348 | 266 | 28 July 2015 |
| NDVI | 10 | 2016 | 194 | 354 | 160 | 28 August 2016 |
| Land GCC | 25 | 2012 | 230 | 295 | 65 | 24 September 2012 |
| Land GCC | 25 | 2013 | 209 | 287 | 78 | 6 September 2013 |
| Land GCC | 25 | 2014 | 194 | 300 | 106 | 6 September 2014 |
| Land GCC | 25 | 2015 | 184 | 310 | 126 | 6 August 2015 |
| Land GCC | 25 | 2016 | 224 | 292 | 68 | 5 September 2016 |
| NDVI | 25 | 2012 | 89 | 356 | 267 | 27 July 2012 |
| NDVI | 25 | 2013 | 199 | 332 | 133 | 14 September 2013 |
| NDVI | 25 | 2014 | 180 | 345 | 165 | 29 August 2014 |
| NDVI | 25 | 2015 | 116 | 317 | 201 | 28 July 2015 |
| NDVI | 25 | 2016 | 202 | 319 | 117 | 28 August 2016 |
References
- Schwartz, M.D.; Betancourt, J.L.; Weltzin, J.F. From Caprio’s lilacs to the USA national phenology network. Front. Ecol. Environ. 2012, 10, 324–327. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kuebler, K.; Bissolli, P.; Braslavska, O.g.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Noormets, A. Phenology of Ecosystem Processes: Applications in Global Change Research; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E.E. The phenology of plant invasions: A community ecology perspective. Front. Ecol. Environ. 2011, 9, 287–294. [Google Scholar] [CrossRef]
- Enquist, C.A.F.; Kellermann, J.L.; Gerst, K.L.; Miller-Rushing, A.J. Phenology research for natural resource management in the united states. Int. J. Biometeorol. 2014, 58, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.M.; Rango, A.; Karl, J.W.; Laney, C.M.; Vivoni, E.R.; Tweedie, C.E. Emerging technological and cultural shifts advancing drylands research and management. Front. Ecol. Environ. 2015, 13, 52–60. [Google Scholar] [CrossRef]
- Richardson, A.D.; Weltzin, J.F.; Morisette, J.T. Integrating Multiscale Seasonal Data for Resource Management. Available online: https://0-doi-org.brum.beds.ac.uk/10.1029/2017EO065709 (accessed on 19 October 2017).
- Melillo, J.M.; Richmond, T.C.; Yohe, G.W. Climate Change Impacts in the United States: The Third National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2014; p. 841.
- US EPA. Climate Change Indicators in the United States, 3rd ed.; Epa 430-r-14-004; 2014. Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-leaf-and-bloom-dates (accessed on 10 September 2017).
- Stiver, S.J.; Rinkes, E.T.; Naugle, D.E.; Makela, P.D.; Nance, D.A.; Karl, J.W. Sage-Grouse Habitat Assessment Framework: Multiscale Habitat Assessment Tool; Bureau of Land Management and Western Association of Fish and Wildlife Agencies: Denver, CO, USA, 2015. [Google Scholar]
- Peterson, E.B. Estimating cover of an invasive grass (Bromus tectorum) using tobit regression and phenology derived from two dates of Landsat ETM+ data. Int. J. Remote Sens. 2005, 26, 2491–2507. [Google Scholar] [CrossRef]
- Horion, S.; Prishchepov, A.V.; Verbesselt, J.; Beurs, K.D.; Tagesson, T.; Fensholt, R. Revealing turning points in ecosystem functioning over the Northern Eurasian agricultural frontier. Glob. Chang. Biol. 2016, 22, 2801–2817. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.H.; Du, P.J.; Feng, L. Phenology-driven land cover classification and trend analysis based on long-term remote sensing image series. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 1142–1156. [Google Scholar] [CrossRef]
- Huete, A.R.; Jackson, R.D. Suitability of spectral indices for evaluating vegetation characteristics on arid rangelands. Remote Sens. Environ. 1987, 23, 213–232. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.Y.; Wang, Z.S.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.Y.; Verfaillie, J.; et al. Using data from Landsat, MODIS, VIIRS and PhenoCams to monitor the phenology of California oak/grass savanna and open grassland across spatial scales. Agric. For. Meteorol. 2017, 237, 311–325. [Google Scholar] [CrossRef]
- Brown, T.B.; Hultine, K.R.; Steltzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; SanClements, M.; et al. Using phenocams to monitor our changing earth: Toward a global phenocam network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- Okin, G.S. The contribution of brown vegetation to vegetation dynamics. Ecology 2010, 91, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Heidebrecht, K.B. Spectral unmixing of vegetation, soil and dry carbon cover in arid regions: Comparing multispectral and hyperspectral observations. Int. J. Remote Sens. 2002, 23, 3939–3958. [Google Scholar] [CrossRef]
- Morisette, J.T.; Richardson, A.D.; Knapp, A.K.; Fisher, J.I.; Graham, E.A.; Abatzoglou, J.; Wilson, B.E.; Breshears, D.D.; Henebry, G.M.; Hanes, J.M.; et al. Tracking the rhythm of the seasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 2009, 7, 253–260. [Google Scholar] [CrossRef]
- Melaas, E.K.; Friedl, M.A.; Richardson, A.D. Multiscale modeling of spring phenology across Deciduous Forests in the Eastern United States. Glob. Chang. Biol. 2016, 22, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.T.; Hufkens, K.; Gray, J.M.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.A.; Richardson, A.D. Evaluating remote sensing of deciduous forest phenology at multiple spatial scales using PhenoCam imagery. Biogeosciences 2014, 11, 4305–4320. [Google Scholar] [CrossRef] [Green Version]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Keenan, T.F.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.A.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M.; et al. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G.; et al. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, K.A.; Wehan, B.L.; Filippa, G.; Huntington, J.L.; Stringham, T.K.; Snyder, D.K. Extracting plant phenology metrics in a great basin watershed: Methods and considerations for quantifying phenophases in a cold desert. Sensors 2016, 16, 1948. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Black, T.A.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S.; et al. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havstad, K.M.; Huennecke, L.F.; Schlesinger, W.H. Structure and Function of a Chihuahuan Desert Ecosystem. The Jornada Basin Long-Term Ecological Research Site; Oxford University Press: Oxford, UK, 2006; p. 465. [Google Scholar]
- Seager, R.; Ting, M.F.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.P.; Harnik, N.; Leetmaa, A.; Lau, N.C.; et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, J. Climate and climatological variations in the jornada basin. In Structure and Function of a Chihuahuan Desert Ecosystem. The Jornada Basin Long-Term Ecological Research Site; Havstad, K.M., Huennecke, L.F., Schlesinger, W.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 44–80. [Google Scholar]
- USDA-NRCS. Ecological Site Information System; National Resource Conservation Service: Lincoln, NE, USA, 2010.
- Gibbens, R.P.; McNeely, R.P.; Havstad, K.M.; Beck, R.F.; Nolen, B. Vegetation changes in the Jornada basin from 1858 to 1998. J. Arid Environ. 2005, 61, 651–668. [Google Scholar] [CrossRef]
- Browning, D.M.; Duniway, M.C.; Laliberte, A.S.; Rango, A. Hierarchical analysis of vegetation dynamics over 71 years: Soil-rainfall interactions in a Chihuahuan Desert ecosystem. Ecol. Appl. 2012, 22, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.R. Phenological patterns of Chihuahuan Desert plants in relation to timing of water availability. J. Ecol. 1983, 71, 427–436. [Google Scholar] [CrossRef]
- Duniway, M.C.; Herrick, J.E.; Monger, H.C. Spatial and temporal variability of plant-available water in calcium carbonate-cemented soils and consequences for arid ecosystem resilience. Oecologia 2010, 163, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Gibbens, R.P.; Lenz, J.M. Root systems of some Chihuahuan Desert plants. J. Arid Environ. 2001, 49, 221–263. [Google Scholar] [CrossRef]
- Denny, E.G.; Gerst, K.L.; Miller-Rushing, A.J.; Tierney, G.L.; Crimmins, T.M.; Enquist, C.A.F.; Guertin, P.; Rosemartin, A.H.; Schwartz, M.D.; Thomas, K.A.; et al. Standardized phenology monitoring methods to track plant and animal activity for science and resource management applications. Int. J. Biometeorol. 2014, 58, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Miller-Rushing, A.J.; Weltzin, J. Phenology as a tool to link ecology and sustainable decision making in a dynamic environment. New Phytol. 2009, 184, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Gitelson, A.A.; Arkebauer, T.J. Modis-based corn grain yield estimation model incorporating crop phenology information. Remote Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Piao, S.; Tan, J.; Chen, A.; Fu, Y.H.; Ciais, P.; Liu, Q.; Janssens, I.A.; Vicca, S.; Zeng, Z.; Jeong, S.-J.; et al. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Gillette, D.; Monger, H.C. Eolian processes on the jornada basin. In Structure and Function of a Chihuahuan Desert Ecosystem. The Jornada Basin Long-Term Ecological Research Site; Havstad, K.M., Huennecke, L.F., Schlesinger, W.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 189–210. [Google Scholar]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; SueWing, I.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. Timesat—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Gherardi, L.A.; Sala, O.E. Enhanced precipitation variability decreases grass- and increases shrub-productivity. Proc. Natl. Acad. Sci. USA 2015, 112, 12735–12740. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.M.; Maynard, J.J.; Karl, J.W.; Peters, D.C. Breaks in modis time series portend vegetation change: Verification using long-term data in an arid grassland ecosystem. Ecol. Appl. 2017, 27, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Hufkens, K.; Keenan, T.F.; Flanagan, L.B.; Scott, R.L.; Bernacchi, C.J.; Joo, E.; Brunsell, N.A.; Verfaillie, J.; Richardson, A.D. Productivity of North American grasslands is increased under future climate scenarios despite rising aridity. Nat. Clim. Chang. 2016, 6, 710–714. [Google Scholar] [CrossRef]
- Munson, S.M.; Long, A.L. Climate drives shifts in grass reproductive phenology across the western USA. New Phytol. 2017, 213, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Munson, S.M.; Muldavin, E.H.; Belnap, J.; Peters, D.P.C.; Anderson, J.P.; Reiser, M.H.; Gallo, K.; Melgoza-Castillo, A.; Herrick, J.E.; Christiansen, T.A. Regional signatures of plant response to drought and elevated temperature across a desert ecosystem. Ecology 2013, 94, 2030–2041. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Klosterman, S.; Toomey, M. Near-surface sensor-derived phenology. In Phenology: An Integrative Science; Schwartz, M.D., Ed.; Springer Science+Business Media: Berlin, Germany, 2013; pp. 413–430. [Google Scholar]
- Petach, A.R.; Toomey, M.; Aubrecht, D.M.; Richardson, A.D. Monitoring vegetation phenology using an infrared-enabled security camera. Agric. For. Meteorol. 2014, 195, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Kao, R.H.; Gibson, C.M.; Gallery, R.E.; Meier, C.L.; Barnett, D.T.; Docherty, K.M.; Blevins, K.K.; Travers, P.D.; Azuaje, E.; Springer, Y.P.; et al. NEON terrestrial field observations: Designing continental-scale, standardized sampling. Ecosphere 2012, 3, 1–17. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking Vegetation phenology across diverse north American biomes using phenocam imagery. Scientific Data 2017. submitted. [Google Scholar]







| Species | Year | SOS | EOS | Duration (# Days) |
|---|---|---|---|---|
| Black grama | 2012 | 87 (7.7) | 308 (3.1) | 221 |
| Black grama | 2013 | 203 (0.0) | 325 (3.8) | 122 |
| Black grama | 2014 | 127 (34.4) | 334 (11.5) | 207 |
| Black grama | 2015 | 89 (0.0) | 355 (0.0) | 266 |
| Black grama | 2016 | 66 (2.7) | 306 (0.0) | 240 |
| Mesquite | 2012 | 114 (4.9) | 324 (0.0) | 210 |
| Mesquite | 2013 | 108 (3.8) | 335 (3.1) | 227 |
| Mesquite | 2014 | 101 (7.8) | 330 (0.0) | 229 |
| Mesquite | 2015 | 109 (15.4) | 340 (7.7) | 231 |
| Mesquite | 2016 | 114 (3.8) | 354 (3.1) | 240 |
| Target | Metric | Threshold | RMSE |
|---|---|---|---|
| Black grama | SOS | 10 | 105.4 |
| Black grama | SOS | 15 | 107.1 |
| Black grama | SOS | 20 | 107.9 |
| Black grama | SOS | 25 | 109.2 |
| Black grama | EOS | 10 | 23.9 |
| Black grama | EOS | 15 | 22.6 |
| Black grama | EOS | 20 | 28.9 |
| Black grama | EOS | 25 | 32.8 |
| Mesquite | SOS | 10 | 8.6 |
| Mesquite | SOS | 15 | 10.6 |
| Mesquite | SOS | 20 | 12.9 |
| Mesquite | SOS | 25 | 13.6 |
| Mesquite | EOS | 10 | 16.3 |
| Mesquite | EOS | 15 | 9.9 |
| Mesquite | EOS | 20 | 8.3 |
| Mesquite | EOS | 25 | 8.2 |
| Metric Method | Metric | Threshold | RMSE |
|---|---|---|---|
| Timesat | SOS | 10 | 63.5 |
| Timesat | SOS | 15 | 47.3 |
| Timesat | SOS | 20 | 57.8 |
| Timesat | SOS | 25 | 36.8 |
| Timesat | EOS | 10 | 44.1 |
| Timesat | EOS | 15 | 46.8 |
| Timesat | EOS | 20 | 39.6 |
| Timesat | EOS | 25 | 34.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, D.M.; Karl, J.W.; Morin, D.; Richardson, A.D.; Tweedie, C.E. Phenocams Bridge the Gap between Field and Satellite Observations in an Arid Grassland Ecosystem. Remote Sens. 2017, 9, 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9101071
Browning DM, Karl JW, Morin D, Richardson AD, Tweedie CE. Phenocams Bridge the Gap between Field and Satellite Observations in an Arid Grassland Ecosystem. Remote Sensing. 2017; 9(10):1071. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9101071
Chicago/Turabian StyleBrowning, Dawn M., Jason W. Karl, David Morin, Andrew D. Richardson, and Craig E. Tweedie. 2017. "Phenocams Bridge the Gap between Field and Satellite Observations in an Arid Grassland Ecosystem" Remote Sensing 9, no. 10: 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9101071