How Reliable Is Structure from Motion (SfM) over Time and between Observers? A Case Study Using Coral Reef Bommies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Surveys
2.2.1. Inter-Individual Variability in Documenting Coral Bommies
2.2.2. Relationship between the Number of Images and Bommie Size
2.2.3. Temporal Variability in Documenting Coral Bommies
2.3. Analyses
2.3.1. Image Processing
2.3.2. Software Measurement Error
2.3.3. Processing Effect
2.3.4. Impact of Coral Morphotype on Error
2.3.5. Inter-Individual Variability in Processing Images for 3D
2.3.6. Temporal Effect
2.3.7. Image Numbers
2.3.8. Predicting Error Margins for Ecological Monitoring
3. Results
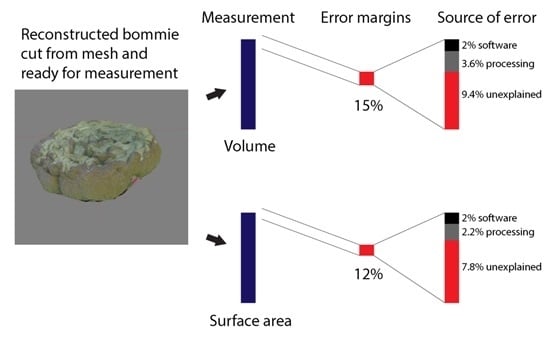
3.1. Software Measurement Error
3.2. Processing Effect
3.3. Morphotype Effect
3.4. Inter-Individual Variability in Processing Images for 3D
3.5. Temporal Effect
3.6. Image Numbers
3.7. Predicting Error Margins for Ecological Monitoring
4. Discussion
4.1 Recommendations for Future Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.M. Incorporating uncertainty in predicting the future response of coral reefs to climate change. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 281–303. [Google Scholar] [CrossRef]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the great barrier reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lafratta, A.; Fromont, J.; Speare, P.; Schönberg, C. Coral bleaching in turbid waters of North-Western Australia. Mar. Freshw. Res. 2016, 68, 65–75. [Google Scholar] [CrossRef]
- Normile, D. El niño’s warmth devastating reefs worldwide. Science 2016, 352, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Cinner, J.E.; Huchery, C.; MacNeil, M.A.; Graham, N.A.; McClanahan, T.R.; Maina, J.; Maire, E.; Kittinger, J.N.; Hicks, C.C.; Mora, C. Bright spots among the world’s coral reefs. Nature 2016, 535, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Berkelmans, R.; Oliver, J. Large-scale bleaching of corals on the great barrier reef. Coral Reefs 1999, 18, 55–60. [Google Scholar] [CrossRef]
- Raoult, V.; David, P.A.; Dupont, S.F.; Mathewson, C.P.; O’Neill, S.J.; Powell, N.N.; Williamson, J.E. Gopros™ as an underwater photogrammetry tool for citizen science. PeerJ 2016, 4, e1960. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Bryson, M.; Bridge, T.; Hustache, J.; Williams, S.B.; Byrne, M.; Figueira, W. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob. Chang. Biol. 2016, 22, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Figueira, W.; Ferrari, R.; Weatherby, E.; Porter, A.; Hawes, S.; Byrne, M. Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Remote Sens. 2015, 7, 16883–16900. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Dartnell, P.; Hatcher, G.A.; Gibbs, A.E. End of the chain? Rugosity and fine-scale bathymetry from existing underwater digital imagery using structure-from-motion (sfm) technology. Coral Reefs 2016, 35, 889–894. [Google Scholar] [CrossRef]
- Lavy, A.; Eyal, G.; Neal, B.; Keren, R.; Loya, Y.; Ilan, M. A quick, easy and non-intrusive method for underwater volume and surface area evaluation of benthic organisms by 3D computer modelling. Methods Ecol. Evol. 2015, 6, 521–531. [Google Scholar] [CrossRef]
- Bennecke, S.; Kwasnitschka, T.; Metaxas, A.; Dullo, W.-C. In situ growth rates of deep-water octocorals determined from 3D photogrammetric reconstructions. Coral Reefs 2016, 35, 1227–1239. [Google Scholar] [CrossRef]
- Young, G.; Dey, S.; Rogers, A.; Exton, D. Cost and time-effective method for multi-scale measures of rugosity, fractal dimension, and vector dispersion from coral reef 3D models. PLoS ONE 2017, 12, e0175341. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Carpenter, R.C. Water flow influences the mechanisms and outcomes of interactions between massive porites and coral reef algae. Mar. Biol. 2015, 162, 459–468. [Google Scholar] [CrossRef]
- Wheeler, S.; Robbins, W.; McIllwain, J. Reef sharks clean up with a novel inshore mutualistic interaction. Coral Reefs 2013, 32, 1089. [Google Scholar] [CrossRef]
- Poray, A.; Carpenter, R. Distributions of coral reef macroalgae in a back reef habitat in moorea, french polynesia. Coral Reefs 2014, 33, 67–76. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Jennings, S.; Polunin, N.V.; Bijoux, J.P.; Robinson, J. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 8425–8429. [Google Scholar] [CrossRef] [PubMed]
- Cocito, S.; Sgorbini, S.; Peirano, A.; Valle, M. 3-D reconstruction of biological objects using underwater video technique and image processing. J. Exp. Mar. Biol. Ecol. 2003, 297, 57–70. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Williamson, J. Availability of two forms of dissolved nitrogen to the coral pocillopora damicornis and its symbiotic zooxanthellae. Mar. Biol. 1999, 133, 561–570. [Google Scholar] [CrossRef]
- Ferrari, R. The hidden structure in coral reefs. Coral Reefs 2017, 36, 445. [Google Scholar] [CrossRef]
- Lucieer, A.; Jong, S.M.D.; Turner, D. Mapping landslide displacements using structure from motion (sfm) and image correlation of multi-temporal UAV photography. Prog. Phys. Geogr. 2014, 38, 97–116. [Google Scholar] [CrossRef]
- Javernick, L.; Brasington, J.; Caruso, B. Modeling the topography of shallow braided rivers using structure-from-motion photogrammetry. Geomorphology 2014, 213, 166–182. [Google Scholar] [CrossRef]
- Wallace, L.; Lucieer, A.; Malenovský, Z.; Turner, D.; Vopěnka, P. Assessment of forest structure using two uav techniques: A comparison of airborne laser scanning and structure from motion (sfm) point clouds. Forests 2016, 7, 62. [Google Scholar] [CrossRef]
- Marteau, B.; Vericat, D.; Gibbins, C.; Batalla, R.J.; Green, D.R. Application of structure-from-motion photogrammetry to river restoration. Earth Surf. Process. Landf. 2017, 42, 503–515. [Google Scholar] [CrossRef]
- Mathews, A.J.; Jensen, J.L. Visualizing and quantifying vineyard canopy lai using an unmanned aerial vehicle (UAV) collected high density structure from motion point cloud. Remote Sens. 2013, 5, 2164–2183. [Google Scholar] [CrossRef]
- Morgenroth, J.; Gomez, C. Assessment of tree structure using a 3d image analysis technique—A proof of concept. Urban For. Urban Green. 2014, 13, 198–203. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Mitra, A.; Castellani, C.; Gentleman, W.C.; Jónasdóttir, S.H.; Flynn, K.J.; Bode, A.; Halsband, C.; Kuhn, P.; Licandro, P.; Agersted, M.D. Bridging the gap between marine biogeochemical and fisheries sciences; configuring the zooplankton link. Prog. Oceanogr. 2014, 129, 176–199. [Google Scholar] [CrossRef]
- Ventura, D.; Bruno, M.; Lasinio, G.J.; Belluscio, A.; Ardizzone, G. A low-cost drone based application for identifying and mapping of coastal fish nursery grounds. Estuar. Coast. Shelf Sci. 2016, 171, 85–98. [Google Scholar] [CrossRef]
- Chirayath, V.; Earle, S.A. Drones that see through waves-preliminary results from airborne fluid lensing for centimetre-scale aquatic conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 237–250. [Google Scholar] [CrossRef]
- Smith, M.; Carrivick, J.; Quincey, D. Structure from motion photogrammetry in physical geography. Prog. Phys. Geor. 2016, 40, 247–275. [Google Scholar] [CrossRef]
- Georgiou, L.; Falter, J.; Trotter, J.; Kline, D.I.; Holcomb, M.; Dove, S.G.; Hoegh-Guldberg, O.; McCulloch, M. pH homeostasis during coral calcification in a free ocean CO2 enrichment (foce) experiment, heron island reef flat, great barrier reef. Proc. Natl. Acad. Sci. USA 2015, 112, 13219–13224. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.I.; Teneva, L.; Schneider, K.; Miard, T.; Chai, A.; Marker, M.; Headley, K.; Opdyke, B.; Nash, M.; Valetich, M. A short-term in situ CO2 enrichment experiment on heron island (GBR). Sci. Rep. 2012, 2, 413. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.E.; Byrnes, E.E.; Clark, J.A.; Connolly, D.M.; Schiller, S.E.; Thompson, J.A.; Tosetto, L.; Martinelli, J.C.; Raoult, V. Ecological impacts and management implications of reef walking on a tropical reef flat community. Mar. Pollut. Bull. 2017, 114, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Heredia, L.; Benzoni, F.; Murphy, E.; Reynaud, E.G. End to end digitisation and analysis of three-dimensional coral models, from communities to corallites. PLoS ONE 2016, 11, e0149641. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.X.; Roelfsema, C.M.; Saunders, M.I.; Phinn, S.R. Measuring coral reef terrain roughness using ‘structure-from-motion’close-range photogrammetry. Geomorphology 2015, 242, 21–28. [Google Scholar] [CrossRef]
- Burns, J.; Delparte, D.; Gates, R.; Takabayashi, M. Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3D ecological characteristics of coral reefs. PeerJ 2015, 3, e1077. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Delparte, D.; Gates, R.; Takabayashi, M. Utilizing underwater three-dimensional modeling to enhance ecological and biological studies of coral reefs. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 61. [Google Scholar] [CrossRef]
- Fonstad, M.A.; Dietrich, J.T.; Courville, B.C.; Jensen, J.L.; Carbonneau, P.E. Topographic structure from motion: A new development in photogrammetric measurement. Earth Surf. Process. Landf. 2013, 38, 421–430. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Measurement error proportional to the mean. BMJ 1996, 313, 106. [Google Scholar] [CrossRef] [PubMed]
- Scheipl, F.; Greven, S.; Kuechenhoff, H. Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Comput. Stat. Data Anal. 2008, 52, 3283–3299. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Manzello, D.P.; Enochs, I.C.; Kolodziej, G.; Carlton, R. Coral growth patterns of montastraea cavernosa and porites astreoides in the florida keys: The importance of thermal stress and inimical waters. J. Exp. Mar. Biol. Ecol. 2015, 471, 198–207. [Google Scholar] [CrossRef]
- Lirman, D.; Schopmeyer, S.; Galvan, V.; Drury, C.; Baker, A.C.; Baums, I.B. Growth dynamics of the threatened caribbean staghorn coral acropora cervicornis: Influence of host genotype, symbiont identity, colony size, and environmental setting. PLoS ONE 2014, 9, e107253. [Google Scholar] [CrossRef] [PubMed]
- Buddemeier, R.W.; Maragos, J.E.; Knutson, D.W. Radiographic studies of reef coral exoskeletons: Rates and patterns of coral growth. J. Exp. Mar. Biol. Ecol. 1974, 14, 179–199. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a caribbean coral reef. Sci. AAAS Wkly. Pap. Ed. 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Van Woesik, R.; De Vantier, L.; Glazebrook, J. Effects of cyclone‘joy’on nearshore coral communities of the great barrier reef. Mar. Ecol. Prog. Ser. 1995, 128, 261–270. [Google Scholar] [CrossRef]
- Vega Thurber, R.L.; Burkepile, D.E.; Fuchs, C.; Shantz, A.A.; McMinds, R.; Zaneveld, J.R. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2014, 20, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Delparte, D.; Kapono, L.; Belt, M.; Gates, R.; Takabayashi, M. Assessing the impact of acute disturbances on the structure and composition of a coral community using innovative 3D reconstruction techniques. Methods Oceanogr. 2016, 15–16, 49–59. [Google Scholar] [CrossRef]
- Holmes, G.; Ortiz, J.; Kaniewska, P.; Johnstone, R. Using three-dimensional surface area to compare the growth of two pocilloporid coral species. Mar. Biol. 2008, 155, 421–427. [Google Scholar] [CrossRef]
- Reichert, J.; Schellenberg, J.; Schubert, P.; Wilke, T. 3D scanning as a highly precise, reproducible, and minimally invasive method for surface area and volume measurements of scleractinian corals. Limnol. Oceanogr. Methods 2016, 14, 518–526. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. A method for determining the surface area of corals. Coral Reefs 1988, 7, 113–116. [Google Scholar] [CrossRef]
- Naumann, M.S.; Niggl, W.; Laforsch, C.; Glaser, C.; Wild, C. Coral surface area quantification–evaluation of established techniques by comparison with computer tomography. Coral Reefs 2009, 28, 109–117. [Google Scholar] [CrossRef]
- Holmes, G. Estimating three-dimensional surface areas on coral reefs. J. Exp. Mar. Biol. Ecol. 2008, 365, 67–73. [Google Scholar] [CrossRef]
- Veal, C.; Carmi, M.; Fine, M.; Hoegh-Guldberg, O. Increasing the accuracy of surface area estimation using single wax dipping of coral fragments. Coral Reefs 2010, 29, 893–897. [Google Scholar] [CrossRef]
- Agudo-Adriani, E.A.; Cappelletto, J.; Cavada-Blanco, F.; Croquer, A. Colony geometry and structural complexity of the endangered species acropora cervicornis partly explains the structure of their associated fish assemblage. PeerJ 2016, 4, e1861. [Google Scholar] [CrossRef] [PubMed]
- Bollard-Breen, B.; Brooks, J.D.; Matthew, R.; Jones, L.; Robertson, J.; Betschart, S.; Kung, O.; Cary, S.C.; Lee, C.K.; Pointing, S.B. Application of an unmanned aerial vehicle in spatial mapping of terrestrial biology and human disturbance in the mcmurdo dry valleys, east antarctica. Polar Biol. 2015, 38, 573. [Google Scholar] [CrossRef]
- Zainuddin, K.; Jaffri, M.; Zainal, M.; Ghazali, N.; Samad, A. Verification test on ability to use low-cost uav for quantifying tree height. In Proceedings of the 2016 IEEE 12th International Colloquium on Signal Processing & Its Applications (CSPA), Malacca City, Malaysia, 4–6 March 2016; pp. 317–321. [Google Scholar]
- Surový, P.; Yoshimoto, A.; Panagiotidis, D. Accuracy of reconstruction of the tree stem surface using terrestrial close-range photogrammetry. Remote Sens. 2016, 8, 123. [Google Scholar] [CrossRef]
- Harwin, S.; Lucieer, A. Assessing the accuracy of georeferenced point clouds produced via multi-view stereopsis from unmanned aerial vehicle (UAV) imagery. Remote Sens. 2012, 4, 1573–1599. [Google Scholar] [CrossRef]






| Weight | True Measurement | Reference Weight 1 | Reference Weight 2 | Reference Weight 3 | Reference Weight 4 | Standard Deviation | Proportion of Error |
|---|---|---|---|---|---|---|---|
| 1 | 50.00 | 51.94 | 49.36 | 49.30 | 1.51 | 0.03 | |
| 2 | 55.00 | 52.95 | 52.26 | 52.20 | 0.41 | 0.01 | |
| 3 | 54.00 | 54.71 | 56.83 | 53.94 | 1.50 | 0.03 | |
| 4 | 54.00 | 54.77 | 56.89 | 54.06 | 1.47 | 0.03 | |
| Mean | 1.22 | 0.02 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoult, V.; Reid-Anderson, S.; Ferri, A.; Williamson, J.E. How Reliable Is Structure from Motion (SfM) over Time and between Observers? A Case Study Using Coral Reef Bommies. Remote Sens. 2017, 9, 740. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9070740
Raoult V, Reid-Anderson S, Ferri A, Williamson JE. How Reliable Is Structure from Motion (SfM) over Time and between Observers? A Case Study Using Coral Reef Bommies. Remote Sensing. 2017; 9(7):740. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9070740
Chicago/Turabian StyleRaoult, Vincent, Sarah Reid-Anderson, Andreas Ferri, and Jane E. Williamson. 2017. "How Reliable Is Structure from Motion (SfM) over Time and between Observers? A Case Study Using Coral Reef Bommies" Remote Sensing 9, no. 7: 740. https://0-doi-org.brum.beds.ac.uk/10.3390/rs9070740