Comparison among Different Gilthead Sea Bream (Sparus aurata) Farming Systems: Activity of Intestinal and Hepatic Enzymes and 13C-NMR Analysis of Lipids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Flesh Composition and Activity of Intestinal Digestive Enzymes
| (mg/g dry tissue) | Semi-intensive | Land based intensive | Sea cages | Wild sea breams |
|---|---|---|---|---|
| Protein | 377.73 ± 4.15 | 585.88 ± 32.82 | 488.57 ± 15.89 | 486.45 ± 15.31 |
| Lipids | 626.00 ± 62.39 | 358.92 ± 30.05 | 414.68 ± 56.52 | 506.27 ± 75.44 |
| Cholesterol | 6.31 ± 0.76 | 9.1 ± 0.11 | 6.05 ± 0.01 | 11.07 ± 0.57 |
| Semi-intensive vs Land based intensive | Semi-intensive vs Sea Cages | Semi-intensive vs Wild Sea Breams | Land based intensive vs Sea Cages | Land based intensive vs Wild sea breams | Sea cages vs Wild sea breams | |
|---|---|---|---|---|---|---|
| Protein | <0.001 | 0.004 | <0.001 | 0.010 | 0.009 | 0.876 |
| Lipids | 0.003 | 0.006 | 0.069 | 0.206 | 0.035 | 0.168 |
| Cholesterol | 0.003 | 0.589 | <0.001 | <0.001 | 0.004 | <0.001 |
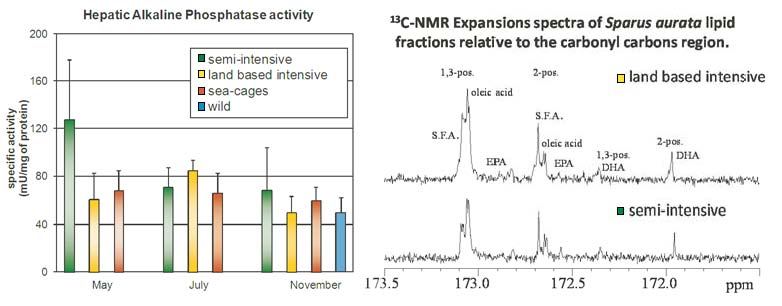
2.2. 13C-NMR Spectra

| Rearing System | Intensive | Semi-Intensive |
|---|---|---|
| 22:6 n-3 | 7.8 ± 2.5 | 6.1 ± 1.7 |
| 20:5 n-3 | 4.1 ± 1.6 | 3.6 ± 0.4 |
| 18:3 n-3 | 8.0 ± 2.7 | 9.7 ± 3.9 |
| 18:1 n-9 | 24.1 ± 0.6* | 30.9 ± 1.9* |
| S.F.A. | 39.5 ± 0.7# | 35.5 ± 1.7# |
| PUFA | 17.0 ± 2.3 | 15.6 ± 4.2 |
| n-3 HUFA | 19.9 ± 1.4 | 19.7 ± 3.0 |
| ratio n-3/n-6 | 1.2 | 1.3 |
| ratio 18:1 n-9/ S.F.A. | 1.6 | 1.2 |


3. Experimental Section
3.1. Sample Preparation andEnzyme Activity Assay
3.2. NMR Measurements
4. Conclusions
Acknowledgements
References
- Basurco, B.; Abellán, E. Finfish species diversification in the context of Mediterranean marine fish farming development. In Marine Finfish Diversification: Current Situation and Prospects in Mediterranean Aquaculture. Options Mediterranéennes FAO, serie B: Etudes et Recherches, no. 24; Abellan, E., Basurco, B., Eds.; CIHEAM: Zaragoza, Spain, 1999; pp. 9–25. [Google Scholar]
- Oliva-Teles, A. Recent advances in European sea bass and gilthead sea bream nutrition. Aquacult. Int. 2000, 8, 477–492. [Google Scholar]
- Aursand, M.; Standal, I.B.; Axelson, D.E. High-resolution 13C nuclear magnetic resonance spectroscopy pattern recognition of fish oil capsules. J. Agric. Food Chem. 2007, 55, 38–47. [Google Scholar]
- Andreotti, G.; Lamanna, R.; Trivellone, E.; Motta, A. 13C NMR spectra of TAG: an easy way to distinguish milks from different animal species. J. Am. Oil Chem. Soc. 2002, 79, 123–127. [Google Scholar]
- Szabó, A.; Fébel, H.; Sugár, L.; Romvári, R. Fatty acid regiodistribution analysis of divergent animal triacylglycerol samples-a possible approach for species differentiation. J. Food Lipids 2007, 14, 62–77. [Google Scholar]
- Vichi, S.M.; Pizzale, L.; Conte, L.S. Stereospecific distribution of fatty acids in triacylglycerols of olive oils. Eur. J. Lipid Sci. Technol. 2007, 109, 72–78. [Google Scholar]
- Vlahov, G. Determination of the 1, 3- and 2-positional distribution of fatty acids in olive oil triacylglycerols by 13C nuclear magnetic resonance spectroscopy. J. AOAC Int. 2006, 89, 1071–1076. [Google Scholar]
- Andrikopoulos, N.K. Chromatographic and spectroscopic methods in the analysis of triacylglycerol species and regiospecific isomers of oils and fats. Crit. Rev. Food Sci. Nutr. 2002, 42, 473–505. [Google Scholar]
- Buchgraber, M.; Ullberth, F.; Emons, H.; Anklam, E. Triacylglycerol profiling by using chromatographic techniques. Eur. J. Lipid Sci. Technol. 2004, 106, 621–648. [Google Scholar]
- Mannina, L.; Dugo, G.; Salvo, F.; Cicero, L.; Ansanelli, G.; Calcagni, C.; Segre, A. Study of the cultivar-composition relationship in Sicilian Olive oils by GC, NMR, and statistical methods. J. Agric. Food Chem. 2003, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D.; Seth, S. A study of the distribution of eicosapentaenoic acid and docosahexaenoic acid between the α and β glycerol chains in fish oils by 13C-NMR spectroscopy. Chem. Phys. Lipids 1994, 72, 119–126. [Google Scholar]
- Aursand, M.; Jørgensen, L.; Grasdalen, H. Positional distribution of ω3 fatty acids in marine lipid triacylglycerols by high resolution 13C nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 1995, 72, 293–297. [Google Scholar]
- Falch, E.; Størset, T.; Aursand, M. Multi-component analysis of marine lipids in fish gonads with emphasis on marine phospholipids using high resolution NMR spectroscopy. Chem. Phys. Lipids 2006, 144, 4–16. [Google Scholar]
- Corcelli, A.; Lattanzio, V.M.T.; Mascolo, G.; Papadia, P.; Fanizzi, F.P. Lipid-protein stoichiometries in a crystalline biological membrane: NMR quantitative analysis of the lipid extract of the purple membrane. J. Lipid Res. 2002, 3, 132–140. [Google Scholar]
- Corcelli, A.; Colella, M.; Mascolo, G.; Fanizzi, F.P.; Kates, M. A novel glycolipid and phospholipid in the purple membrane. Biochemistry 2000, 39, 3318–3326. [Google Scholar]
- Lien, E.L. The role of fatty-acid composition and positional distribution in fat-absorption in infants. J. Pediatr. 1994, 125, S62–S68. [Google Scholar]
- Lien, E.L.; Boyle, F.G.; Yuhas, R.; Tomarelli, R.M.; Quinlan, P. The effect of triglyceride positional distribution on fatty acid absorption in rats. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 167–174. [Google Scholar]
- Standal, I.B.; Axelson, D.E.; Aursand, M. Differentiation of fish oils according to species by 13C-NMR regiospecific analyses of triacyglycerols. J. Am. Oil Chem. Soc. 2009, 86, 401–407. [Google Scholar]
- Del Coco, L.; Papadia, P.; De Pascali, S.A.; Bressani, G.; Storelli, C.; Zonno, V.; Fanizzi, F.P. 13C NMR Spectroscopy and quantitative analysis of lipids from fish: comparison between different farming systems. In Proceedings of International Conference on FOOD-OMICS, Cesena, Italy, May 28–29, 2009.
- Gunstone, F.D. High resolution NMR studies of fish oils. Chem. Phys. Lipids 1991, 59, 83–89. [Google Scholar]
- Gunstone, F.D. High resolution 13C NMR. A technique for the study of lipid structure and composition. Prog. Lipid Res. 1994, 33, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Medina, I.; Auborg, P.; Paolillo, L.; Addeo, F. Quantitative high resolution 13C NMR analysis of lipids extracted from the white muscle of Atlantic Tuna (Thunnus Alalunga). J. Agric. Food Chem. 1993, 41, 1247–1253. [Google Scholar] [CrossRef]
- Siddiqui, N.; Sim, J.; Grootveld, M. Multicomponent analysis of encapsulated marine oil supplements using high resolution 1H and 13C NMR techniques. J. Lipid Res. 2003, 44, 2406–2427. [Google Scholar] [CrossRef] [PubMed]
- Diehl, W. High resolution NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2001, 103, 830–834. [Google Scholar]
- Gunstone, F.D. 13C NMR spectra of some synthetic glycerol esters alone and as a mixture. Chem. Phys. Lipids 1990, 56, 195–199. [Google Scholar]
- Guillen, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar]
- Wollenberg, K.F. Quantitative high resolution carbon-13 nuclear magnetic resonance of the olefinic and carbonyl carbons of edible vegetable oils. J. Am. Oil Chem. Soc. 1990, 67, 487–494. [Google Scholar]
- Hidalgo, F.J.; Zamora, R. Edible oil analysis by high-resolution nuclear magnetic resonance spectroscopy: recent advances and future perspectives. Trends Food Sci. Technol. 2003, 14, 499–506. [Google Scholar]
- Mannina, L.; Sobolev, A.P.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444. [Google Scholar]
- Orban, E.; Di Lena, G.; Ricelli, A.; Paoletti, I.; Casini, L.; Gambelli, R.; Caproni, R. Quality characteristics of sharpsnout sea bream (Diplodus puntazzo) from different intensive rearing systems. Food Chem. 2000, 70, 27–32. [Google Scholar] [CrossRef]
- Storelli, C.; Vilella, S.; Cassano, G. Sodium-dependent D-glucose and L-alanine transport in eel intestinal brush border membrane vesicles. Am. J. Physiol. 1986, 251, 463–469. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Orban, E.; Nevigato, T.; Di Lena, G.; Casini, L. Differentiation in the lipid quality of wild and farmed seabass (Dicentrarchus labrax) and gilthead seabream (Spaurus Aurata). J. Food Sci. 2003, 68, 128–132. [Google Scholar] [CrossRef]
- Rueda, F.M.; Hernandez, M.; Egea, M.A.; Aguado, F.; Garcia, B.; Martinez, F.J. Differences in tissue fatty acid composition between reared and wild sharpsnout sea bream, Diplodus puntazzo (Cetti, 1977). Br. J. Nutr. 2001, 86, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Hernandez, M.; Egea, M.; Garcia, B. Effect of feeding rate on fatty acid composition of sharpsnout seabream (Diplodus puntazzo). Aquacolt. Nutr. 2004, 10, 301–308. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Del Coco, L.; Papadia, P.; De Pascali, S.A.; Bressani, G.; Storelli, C.; Zonno, V.; Fanizzi, F.P. Comparison among Different Gilthead Sea Bream (Sparus aurata) Farming Systems: Activity of Intestinal and Hepatic Enzymes and 13C-NMR Analysis of Lipids. Nutrients 2009, 1, 291-301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu1020291
Del Coco L, Papadia P, De Pascali SA, Bressani G, Storelli C, Zonno V, Fanizzi FP. Comparison among Different Gilthead Sea Bream (Sparus aurata) Farming Systems: Activity of Intestinal and Hepatic Enzymes and 13C-NMR Analysis of Lipids. Nutrients. 2009; 1(2):291-301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu1020291
Chicago/Turabian StyleDel Coco, Laura, Paride Papadia, Sandra A. De Pascali, Giorgia Bressani, Carlo Storelli, Vincenzo Zonno, and Francesco Paolo Fanizzi. 2009. "Comparison among Different Gilthead Sea Bream (Sparus aurata) Farming Systems: Activity of Intestinal and Hepatic Enzymes and 13C-NMR Analysis of Lipids" Nutrients 1, no. 2: 291-301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu1020291