Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation
Abstract
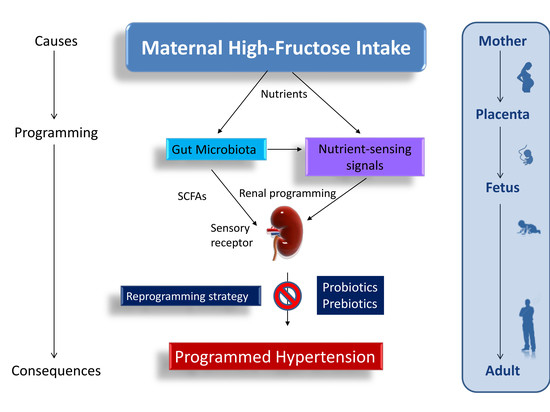
:1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Gas Chromatography-Flame Ionization Detector (GC-FID)
2.3. Gut Microbiota Profiling
2.4. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. Morphological Features and Blood Pressures
3.2. Short Chain Fatty Acids and Nutrient-Sensing Signals
3.3. Gut Microbiota Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizkalla, S.W. Health implications of fructose consumption: A review of recent data. Nutr. Metab. 2010, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regnault, T.R.; Gentili, S.; Sarr, O.; Toop, C.R.; Sloboda, D.M. Fructose, pregnancy and later life impacts. Clin. Exp. Pharmacol. Physiol. 2013, 40, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Galla, S.; Chakraborty, S.; Cheng, X.; Yeo, J.; Mell, B.; Zhang, H.; Mathew, A.V.; Vijay-Kumar, M.; Joe, B. Disparate effects of antibiotics on hypertension. Physiol. Genom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Renal Physiol. 2013, 305, F439–F444. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.C.; Widmer, A.; Baccigalupi, L.; Ricca, E.; Iossa, S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS ONE 2015, 10, e0134893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, H.K. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats. J. Nutr. 1998, 128, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- De Waard, R.; Garssen, J.; Bokken, G.C.; Vos, J.G. Antagonistic activity of Lactobacillus casei strain shirota against gastrointestinal Listeria monocytogenes infection in rats. Int. J. Food Microbiol. 2002, 73, 93–100. [Google Scholar] [CrossRef]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef] [PubMed]
- Grigore, D.; Ojeda, N.B.; Alexander, B.T. Sex differences in the fetal programming of hypertension. Gend. Med. 2008, 5, S121–S132. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-l-arginine-methyl ester (l-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, J.Y.; Hsu, C.N. Maternal fructose intake affects transcriptome changes and programmed hypertension in offspring in later life. Nutrients 2016, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Szeto, I.M.; Makinen, K.; Gao, Q.; Wang, J.; Qin, L.Q.; Zhao, Y. Effect of probiotic fermented milk on blood pressure: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- De Wiele, T.V.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 51, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Lee, W.C.; Wu, K.; Leu, S.; Chan, J.Y.H. Maternal high fructose intake increases the vulnerability to post-weaning high-fat diet induced programmed hypertension in male offspring. Nutrients 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Veličković, N.; Teofilović, A.; Ilić, D.; Djordjevic, A.; Vojnović Milutinović, D.; Petrović, S.; Preitner, F.; Tappy, L.; Matić, G. Modulation of hepatic inflammation and energy-sensing pathways in the rat liver by high-fructose diet and chronic stress. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. AMP-activated protein kinase as a reprogramming strategy for hypertension and kidney disease of developmental origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2018, 42, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Wu, K.L.H.; Leu, S.; Tain, Y.L. Translational insights on developmental origins of metabolic syndrome: Focus on fructose consumption. Biomed. J. 2018, 41, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. SPRING Trial Group. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; Gerber, G.K.; Roeselers, G.; Delaney, M.; DuBois, A.; Liu, Q.; Belavusava, V.; Yeliseyev, V.; Houseman, A.; Onderdonk, A.; et al. Dynamics of the microbiota in response to host infection. PLoS ONE 2014, 9, e95534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked pathophysiological alterations in the gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef] [PubMed]





| Gene Sympol | Gene Name | Forward | Reverse |
|---|---|---|---|
| Sirt4 | Sirtuin-4 | 5′-ccctttggaccatgaaaaga-3′ | 5′-cggatgaaatcaatgtgctg-3′ |
| Prkaa2 | AMP-activated protein kinase, subunit-α2 | 5′-agctcgcagtggcttatcat-3′ | 5′-ggggctgtctgctatgagag-3′ |
| Prkab2 | AMP-activated protein kinase, subunit-β2 | 5′-cagggccttatggtcaagaa-3′ | 5′-cagcgcatagagatggttca-3′ |
| Prkag2 | AMP-activated protein kinase, subunit-γ2 | 5′-gtgtgggagaagctctgagg-3′ | 5′-agaccacacccagaagatgc-3′ |
| Ppara | Peroxisome proliferator-activated receptor-α | 5′-agaagttgcaggaggggatt-3′ | 5′-ttcttgatgacctgcacgag-3′ |
| Pparrb | Peroxisome proliferator-activated receptor-β | 5′-gatcagcgtgcatgtgttct-3′ | 5′-cagcagtccgtctttgttga-3′ |
| Pparg | Peroxisome proliferator-activated receptor-γ | 5′-ctttatggagcctaagtttgagt-3′ | 5′-gttgtcttggatgtcctcg-3′ |
| Ppargc1a | Peroxisome proliferator-activated receptor-γ coactivator 1-α | 5′-cccattgagggctgtgatct-3′ | 5′-tcagtgaaatgccggagtca-3′ |
| Ffar3 | Free Fatty Acid Receptor 3 | 5′-tgaccatttcggacctgctt-3′ | 5′-tgggtaggctacgctcagaa-3′ |
| Ffar2 | Free Fatty Acid Receptor 2 | 5′-gctgtggtgttcagttccct-3′ | 5′-gtttgactcccacccctgtc-3′ |
| Olfr78 | Olfactory receptor 78 | 5′-accggtatgtggctatctgc-3′ | 5′-gtgggagagcacattggagt-3′ |
| R18S | 18S rRNA | 5′-gccgcggtaattccagctcca-3′ | 5′-cccgcccgctcccaagatc-3′ |
| Control | HF | HF+Probiotic | HF+Prebiotic | |
|---|---|---|---|---|
| N = 8 | N = 8 | N = 7 | N = 8 | |
| Body Weight (BW) (g) | 452 ± 12 | 448 ± 10 | 457 ± 6 | 375 ± 3 *#† |
| Left Kidney Weight (g) | 1.69 ± 0.06 | 1.76 ± 0.04 | 1.8 ± 0.04 | 1.68 ± 0.07 |
| Left Kidney Weight/100 g BW | 0.37 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.45 ± 0.02 *#† |
| Systolic Blood Pressure (mmHg) | 141 ± 1 | 154 ± 1 * | 145 ± 2 # | 144 ± 1 # |
| Control | HF | HF+Probiotic | HF+Prebiotic | |
|---|---|---|---|---|
| N = 8 | N = 8 | N = 7 | N = 8 | |
| Acetate (μM) | 12.5 ± 0.4 | 20.3 ± 1.2 * | 12.3 ± 1.1 # | 18.6 ± 0.7 |
| Butyrate (μM) | 5.72 ± 0.38 | 8.56 ± 0.87 | 4.4 ± 0.72 | 7.01 ± 0.4 |
| Propionate (μM) | 0.82 ± 0.04 | 0.96 ± 0.08 | 0.87 ± 0.07 | 2.39 ± 0.3 *#† |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Lin, Y.-J.; Hou, C.-Y.; Tain, Y.-L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091229
Hsu C-N, Lin Y-J, Hou C-Y, Tain Y-L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients. 2018; 10(9):1229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091229
Chicago/Turabian StyleHsu, Chien-Ning, Yu-Ju Lin, Chih-Yao Hou, and You-Lin Tain. 2018. "Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation" Nutrients 10, no. 9: 1229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091229