Chemical Composition and In Vitro Bioaccessibility of Antioxidant Phytochemicals from Selected Edible Nuts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Phytochemical Composition
2.2.1. Chemical Composition
2.2.2. Sample Fractionation
2.2.3. Fatty Acids
2.2.4. Tocols (T + T3)
2.2.5. Carotenoids
2.2.6. Total Lipophilic and Hydrophilic Phenolic Compounds
2.2.7. Flavonoids and Condensed Tannins
2.3. In Vitro Bioaccessibility
2.3.1. Static In Vitro Digestion Method
2.3.2. Apparent Bioaccessibility
2.4. Statistical Analysis and Chemometrics
3. Results
3.1. Chemical Composition
3.2. Antioxidant Phytochemicals
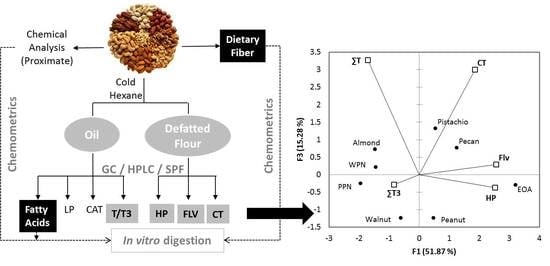
3.3. Principal Component Analysis (PCA)
3.4. Apparent Bioaccessibility
4. Discussion
4.1. Chemical Composition
4.2. Antioxidant Phytochemicals
4.3. Apparent Bioaccessibility
4.4. Chemometrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Therap. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Barrón, J.; de la Rosa, L.A.; Wall-Medrano, A.; Álvarez-Parrilla, E.; Astiazarán-García, H.; Robles-Zepeda, R.E. Efectividad y principales mecanismos anticancerígenos de tocotrienoles en líneas celulares malignas. Rev. Mex. Cienc. Farm. 2017, 48, 16–27. [Google Scholar]
- De Souza, R.; Schincaglia, R.; Pimentel, G.; Mota, J. Nuts and human health outcomes: A systematic review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Parrilla, E.; Urrea-López, R.; de la Rosa, L.A. Bioactive components and health effects of pecan nuts and their byproducts: A review. J. Food Bioact. 2018, 1, 56–92. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Ávila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, Y.; O’Brien, N. Bioaccessibility, cellular uptake and transepithelial transport of α-tocopherol and retinol from a range of supplemented foodstuffs assessed using the Caco-2 cell model. Int. J. Food Sci. Technol. 2010, 45, 1436–1442. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Panozzo, A.; Lemmens, L.; Van Loey, A.; Manzocco, L.; Nicoli, M.C.; Hendrickx, M. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Reguant, J.; Romero, M.P.; Macia, A.; Motilva, M.J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Isbell, T.A.; Mund, M.S.; Evangelista, R.L.; Dierig, D.A. Method for analysis of fatty acid distribution and oil content on a single Lesquerella fendleri seed. Ind. Crops Prod. 2008, 28, 231–236. [Google Scholar] [CrossRef]
- Núñez-Gastélum, J.A.; González-Fernández, R.; Hernández-Herrera, A.; Campas-Baypoli, O.N.; Rodríguez-Ramirez, R.; Lobo-Galo, N.; Valero-Galván, J. Morphological characteristics, chemical composition and antioxidant activity of seeds by four wild Opuntia species from North of Mexico. J. Prof. Assoc. Cactus Dev. 2018, 20, 23–33. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef] [Green Version]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin–Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Gaytán-Martínez, M.; Mendoza-Díaz, S.O.; González-Aguilar, G.A.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Wall-Medrano, A. In vitro digestibility of phenolic compounds from edible fruits: Could it be explained by chemometrics? Int. J. Food Sci. Technol. 2017, 52, 2040–2048. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Drca, N.; Björck, M.; Bäck, M.; Wolk, A. Nut consumption and incidence of seven cardiovascular diseases. Heart 2018, 104, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef]
- Ahuja, J.K.A.; Montville, J.B.; Omolewa-Tomobi, G.; Heendeniya, K.Y.; Martin, C.L.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A.J. USDA Food and Nutrient Database for Dietary Studies, 5.0–Documentation and User Guide; U.S. Department of Agriculture ARS, Food Surveys Research Group: Beltsville, MD, USA, 2012.
- Valero-Galván, J.; Reyna-González, M.; Chico-Romero, P.A.; Martínez-Ruiz, N.D.R.; Núñez-Gastélum, J.A.; Monroy-Sosa, A.; Ruiz, M.E.; González Fernández, R. Seed characteristics and nutritional composition of pine nut from five populations of P. cembroides from the states of Hidalgo and Chihuahua, Mexico. Molecules 2019, 24, 2057. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Al-Mahasneh, M.A.; Alhamad, M.N.; Alrababah, M.A.; Muhammad, A.H. The physicochemical composition of acorns for two mediterranean Quercus species. Jordan J. Agric. Sci. 2008, 4, 131–137. [Google Scholar]
- Rakić, S.; Povrenović, D.; Tešević, V.; Simić, M.; Maletić, R. Oak acorn, polyphenols and antioxidant activity in functional food. J. Food Eng. 2006, 74, 416–423. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L. Tree nut consumption is associated with a better nutrient adequacy and diet quality in adults: National Health and Nutrition Examination Survey 2005–2010. Nutrients 2015, 7, 595–607. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Pérez-Heras, A.; Ros, E. Dietary fibre, nuts and cardiovascular diseases. Br. J. Nutr. 2006, 96, S45–S51. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Khalili Moghadam, S.; Zadeh Vakili, A.; Azizi, F. A prospective study of different types of dietary fiber and risk of cardiovascular disease: Tehran lipid and glucose study. Nutrients 2016, 8, 686. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Johnson, G.H.; Fritsche, K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: A systematic review of randomized controlled trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041. [Google Scholar] [CrossRef]
- Mölenberg, F.J.; de Goede, J.; Wanders, A.J.; Zock, P.L.; Kromhout, D.; Geleijnse, J.M. Dietary fatty acid intake after myocardial infarction: A theoretical substitution analysis of the Alpha Omega Cohort. Am. J. Clin. Nutr. 2017, 106, 895–901. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Liu, X.; Miao, X.; Wu, D.; Liu, C.; Fang, L.; Liu, J.; Min, W. Purification and identification of ACE-inhibiting peptides from wild pine nut peptide fractions (PNPF). Eur. Food Res. Technol. 2018, 244, 979–988. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Robbins, K.S.; Shin, E.C.; Shewfelt, R.L.; Eitenmiller, R.R.; Pegg, R.B. Update on the healthful lipid constituents of commercially important tree nuts. J. Agric. Food Chem. 2011, 59, 12083–12092. [Google Scholar] [CrossRef]
- Mukai, K.; Tokunaga, A.; Itoh, S.; Kanesaki, Y.; Ohara, K.; Nagaoka, S.I.; Abe, K. Structure—Activity Relationship of the Free-Radical-Scavenging Reaction by Vitamin E (α-, β-, γ-, δ-Tocopherols) and Ubiquinol-10: pH Dependence of the Reaction Rates. J. Phys. Chem. 2007, 111, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Radhakrishnan, A.K. Tocotrienol research: Past into present. Nutr. Rev. 2012, 70, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Özrenk, K.; Javidipour, I.; Yarilgac, T.; Balta, F.; Gündoğdu, M. Fatty acids, tocopherols, selenium and total carotene of pistachios (P. vera L.) from Diyarbakır (Southestern Turkey) and walnuts (J. regia L.) from Erzincan (Eastern Turkey). Food Sci. Technol. Int. 2012, 18, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Wall-Medrano, A.; Olivas-Aguirre, F.J. Antioxidant phytochemicals in cancer prevention and therapy—An update. In Functional Foods in Cancer Prevention and Therapy, 1st ed.; Kabir, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, D.; García-Torres, S.; de Vaca, M.C.; Vázquez, F.M.; Cava, R. Acorns (Quercus rotundifolia L.) and grass as natural sources of antioxidants and fatty acids in the “montanera” feeding of Iberian pig: Intra-and inter-annual variations. Food Chem. 2011, 124, 997–1004. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef] [PubMed]
- PopoviT, B.M.; Štajner, D.; Cdero, R.; OrloviT, S.; GaliT, Z. Antioxidant characterization of oak extracts combining spectrophotometric assays and chemometrics. Neurodegener. Dis. 2013, 12, 13. [Google Scholar]
- Liu, L.; Jin, C.; Zhang, Y. Lipophilic phenolic compounds (Lipo-PCs): Emerging antioxidants applied in lipid systems. RSC Adv. 2014, 4, 2879–2891. [Google Scholar] [CrossRef]
- Urness, P.J.; McCulloch, C.Y. Nutritional value of seasonal deer diets (Part III). In Special Report 3. Deer Nutrition in Arizona Chaparral and Desert Habitats; Game and Fish Department: Phoenix, AZ, USA, 1973; pp. 53–68. [Google Scholar]
- Bainbridge, D.A. The use of acorns for food in California: Past, present, future. In Proceedings of the Symposium on Multiple-Use Management of California’s Hardwood Resources, 1st ed.; Plumb, T.R., Pillsbury, N.H., Eds.; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: San Luis Obispo, CA, USA, 1986; pp. 453–458. [Google Scholar]
- Vázquez-Flores, A.A.; Álvarez-Parrilla, E.; López-Díaz, J.A.; Wall-Medrano, A.; De la Rosa, L.A. Taninos hidrolizables y condensados: Naturaleza química, ventajas y desventajas de su consumo. Tecnociencia 2012, 6, 84–93. [Google Scholar]
- Rossi, M.; Edefonti, V.; Parpinel, M.; Lagiou, P.; Franchi, M.; Ferraroni, M.; Decarli, A.; Zucchetto, A.; Serraino, D.; Dal Maso, L.; et al. Proanthocyanidins and other flavonoids in relation to endometrial cancer risk: A case–Control study in Italy. Br. J. Cancer 2013, 109, 1914. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Ifie, I.; Gonzalez-Aguilar, G.A.; Roopchand, D.E. The gastrointestinal tract as prime site for cardiometabolic protection by dietary polyphenols. Adv. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef]
- Spiller, R.; Marciani, L. Intraluminal impact of food: New insights from MRI. Nutrients 2019, 11, 1147. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Lucini, L. Bioaccessibility of phenolic compounds following in vitro large intestine fermentation of nuts for human consumption. Food Chem. 2018, 245, 633–640. [Google Scholar] [CrossRef]
- Ellis, P.R.; Kendall, C.W.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalari, G.; Faulks, R.M.; Rich, G.T.; Lo Turco, V.; Picout, D.R.; Lo Curto, R.B. Release of protein, lipid, and vitamin E from almond seeds during digestion. J. Agric. Food Chem. 2008, 56, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lopez-Sanchez, P.; Gidley, M.J. Cellular barriers in apple tissue regulate polyphenol release under different food processing and in vitro digestion conditions. Food Funct. 2019, 10, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Blumberg, J.B.; Chen, C.Y.O. Quantification and bioaccessibility of California pistachio bioactives. J. Agric. Food Chem. 2014, 62, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Krauß, S.; Darwisch, V.; Vetter, W. Occurrence of tocopheryl fatty acid esters in vegetables and their non-digestibility by artificial digestion juices. Sci. Rep. 2018, 8, 7657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plundrich, N.J.; White, B.L.; Dean, L.L.; Davis, J.P.; Foegeding, E.A.; Lila, M.A. Stability and immunogenicity of hypoallergenic peanut protein–polyphenol complexes during in vitro pepsin digestion. Food Funct. 2015, 6, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.; Novotny, J. Metabolizable energy from cashew nuts is less than that predicted by atwater factors. Nutrients 2019, 11, 33. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Stuetz, W.; Schlörmann, W.; Glei, M. B-vitamins, carotenoids and α-/γ-tocopherol in raw and roasted nuts. Food Chem. 2017, 221, 222–227. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Carrillo, C.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Swetha, M.P.; Radha, C.; Muthukumar, S.P. Bioaccessibility and bioavailability of Moringa oleifera seed flour polyphenols. J. Food Meas. Charact. 2018, 12, 1917–1926. [Google Scholar] [CrossRef]
- López de Felipe, F.; de Las Rivas, B.; Muñoz, R. Bioactive compounds produced by gut microbial tannase: Implications for colorectal cancer development. Front. Microbiol. 2014, 5, 684. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharm. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Edible Nut | α | β | γ | δ |
|---|---|---|---|---|
| Tocopherols (T) | ||||
| Almond (ALM) | 124.9 ± 4.8 a | 16.8 ± 0.4 a | 14.5 ± 0.0 d | -- |
| Emory oak acorn (EOA) | 10.0 ± 0.1 e | 15.2 ± 0.6 b | 55.9 ± 4.7 c | 0.2 ± 0.0 c |
| Pecan (PEC) | 17.1 ± 0.1 d | 18.0 ± 0.3 b | 56.9 ± 2.8 c | 0.02 ± 0.0 c |
| Pistachio (PIS) | 15.2 ± 0.1 d | 15.7 ± 0.4 a | 82.1 ± 6.2 b | 0.4 ± 0.0 b |
| Peanut (PNT) | 13.4 ± 0.4 d,e | 11.4 ± 0.1 c | 22.8 ± 0.7 d | 0.5 ± 0.0 b |
| Pink pine nut (PPN) | 43.9 ± 2.3 b | 4.5 ± 0.04 d | 83.6 ± 1.7 b | 0.3 ± 0.0 c |
| White pine nut (WPN) | 24.2 ± 0.6 c | 5.2 ± 0.0 d | 108.6 ± 1.3 a | 0.2 ± 0.0 c |
| Walnut (WNT) | -- | 15.4 ± 0.2 b | 58.1 ± 1.3 c | 1.7 ± 0.1 a |
| Tocotrienols (T3) | ||||
| ALM | -- | -- | -- | -- |
| EOA | 0.2 ± 0.0 c | 0.6 ± 0.1 a | 1.7 ± 0.1 b | 3.3 ± 0.1 a |
| PEC | 0.3 ± 0.0 c | 0.1 ± 0.0 c | 0.3 ± 0.2 d | 0.3 ± 0.0 b |
| PIS | 0.4 ± 0.1 c | 0.3 ± 0.1 b | 2.0 ± 0.1 a | 0.2 ± 0.0 b |
| PNT | -- | 0.5 ± 0.0 b | 0.1 ± 0.0 e | -- |
| PPN | 20.4 ± 1.0 a | 0.2 ± 0.0 b,c | 1.3 ± 0.1 a,b | 0.3 ± 0.0 b |
| WPN | 2.2 ± 0.3 b | 0.1 ± 0.0 c | 1.1 ± 0.1 c | 0.2 ± 0.0 b |
| WNT | -- | 0.1 ± 0.0 c | -- | -- |
| Edible Nut | HP (mg GAE) | Flv (mg CE) | CT (mg CE) | LP (mg GAE) | CAR (mg/kg) |
|---|---|---|---|---|---|
| Almond (ALM) | 727 ± 37 e | 188 ± 14 d | 85 ± 2 d | 812 ± 57 b,c | 0.01 ± 0.07 d |
| Emory oak acorn (EOA) | 12,896 ± 390 a | 1833 ± 40 a | 460 ± 16 a | 3.3 ± 11 e | 0.16 ± 0.01 b |
| Pecan (PEC) | 3990 ± 111 b | 814 ± 18 b | 348 ± 23 b | 866 ± 33 b | 0.15 ± 0.07 b |
| Pistachio (PIS) | 2530 ± 30 c | 557 ± 41 c | 328 ± 18 b | 555 ± 5 d | 0.17 ± 0.06 b |
| Peanut (PNT) | 2715 ± 77 c | 406 ± 30 c | 164 ± 10 c | 318 ± 2 e | 0.02 ± 0.01 d |
| Pink pine nut (PPN) | 533 ± 74 e,f | 231 ± 17 d | 14 ± 5 e,f | 1219 ± 56 a | 0.05 ± 0.00 d |
| White pine nut (WPN) | 818 ± 20 e | 142 ± 7 d | 38 ± 8 e | 746 ± 13 c | 0.04 ± 0.00 d |
| Walnut (WNT) | 1407 ± 28 d | 169 ± 3.3 d | 115 ± 8 d | 755 ± 23 c | 0.38 ± 0.05 a |
| Model | Matrix’s Phytochemical 1 | β1 | β2 | β3 | R2 | p |
|---|---|---|---|---|---|---|
| T-BA | ||||||
| 1 | TF | 0.01 | - | - | 0.09 | >0.05 |
| 2 | TF + MUFAs | −0.014 | 0.057 | - | 0.10 | <0.03 |
| T3-BA | ||||||
| 1 | PUFAs | −0.002 | - | - | 0.36 | <0.03 |
| 2 | PUFAs + T3 | −0.0003 | 0.32 | - | 0.94 | <0.0001 |
| 3 | PUFAs + T3 + DF | 0.004 | 0.39 | 0.009 | 0.98 | <0.0001 |
| HP-BA | ||||||
| 1 | TF | −0.52 | - | - | 0.78 | <0.0001 |
| 2 | TF + DF | −0.50 | −0.27 | - | 0.94 | <0.0001 |
| Flv-BA | ||||||
| 1 | Flv | 0.022 | - | - | 0.36 | <0.02 |
| 2 | Flv + DF | 0.019 | −0.036 | - | 0.45 | <0.03 |
| 3 | Flv + DF + TF | 0.10 | 0.11 | 0.33 | 0.99 | <0.001 |
| CT-BA | ||||||
| 1 | CT | 0.008 | - | - | 0.980 | <0.001 |
| 2 | CT + DF | 0.008 | −0.008 | - | 0.989 | <0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens-Barrón, J.C.; de la Rosa, L.A.; Wall-Medrano, A.; Álvarez-Parrilla, E.; Rodríguez-Ramirez, R.; Robles-Zepeda, R.E.; Astiazaran-García, H. Chemical Composition and In Vitro Bioaccessibility of Antioxidant Phytochemicals from Selected Edible Nuts. Nutrients 2019, 11, 2303. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102303
Stevens-Barrón JC, de la Rosa LA, Wall-Medrano A, Álvarez-Parrilla E, Rodríguez-Ramirez R, Robles-Zepeda RE, Astiazaran-García H. Chemical Composition and In Vitro Bioaccessibility of Antioxidant Phytochemicals from Selected Edible Nuts. Nutrients. 2019; 11(10):2303. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102303
Chicago/Turabian StyleStevens-Barrón, Jazmín C., Laura A. de la Rosa, Abraham Wall-Medrano, Emilio Álvarez-Parrilla, Roberto Rodríguez-Ramirez, Ramón E. Robles-Zepeda, and Humberto Astiazaran-García. 2019. "Chemical Composition and In Vitro Bioaccessibility of Antioxidant Phytochemicals from Selected Edible Nuts" Nutrients 11, no. 10: 2303. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102303