Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes
Abstract
:1. Cyclitols
2. Myo-Inositol
3. D-Chiro-Inositol
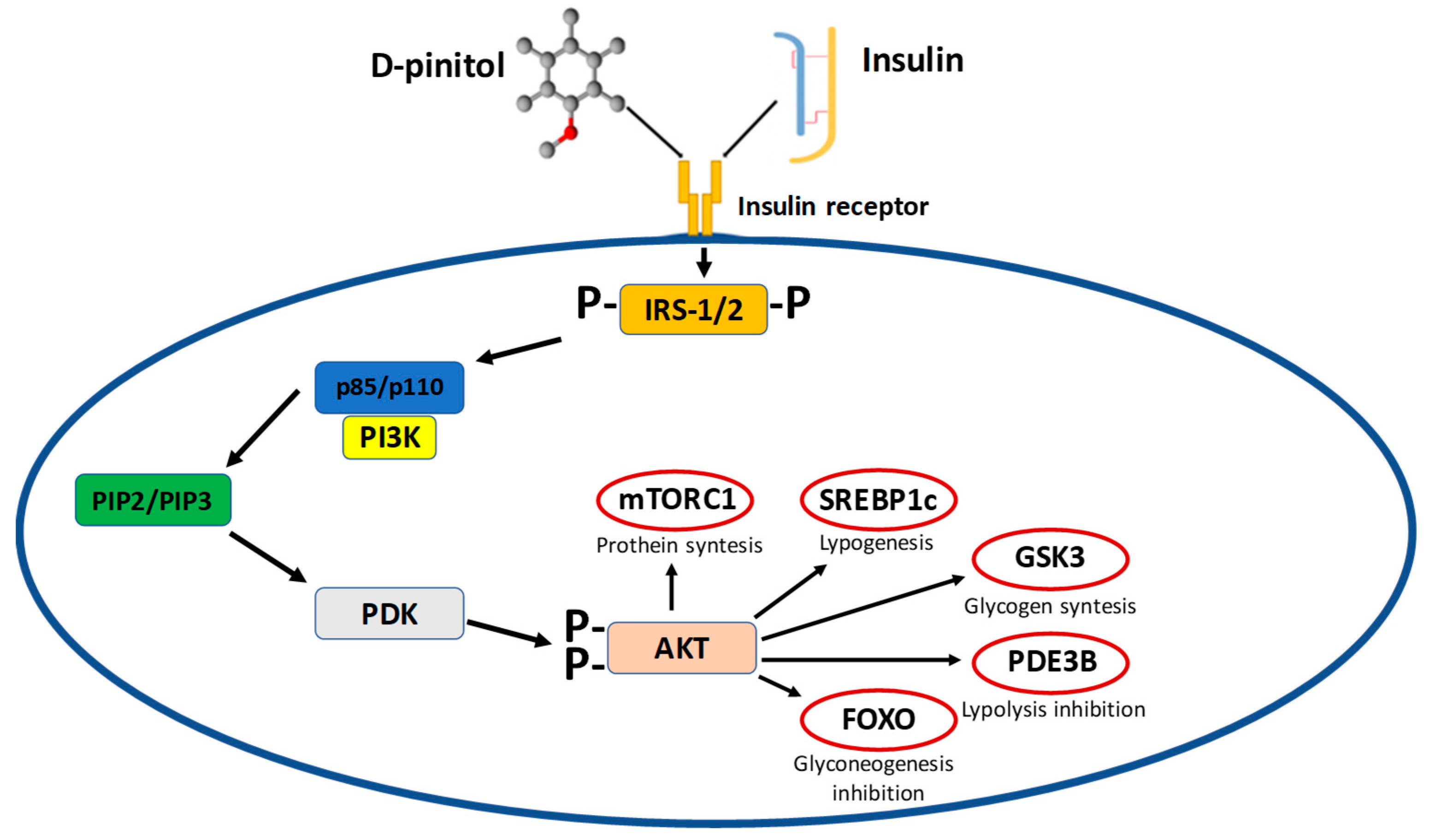
4. D-Pinitol
5. Metabolic Syndrome
6. Diabetes
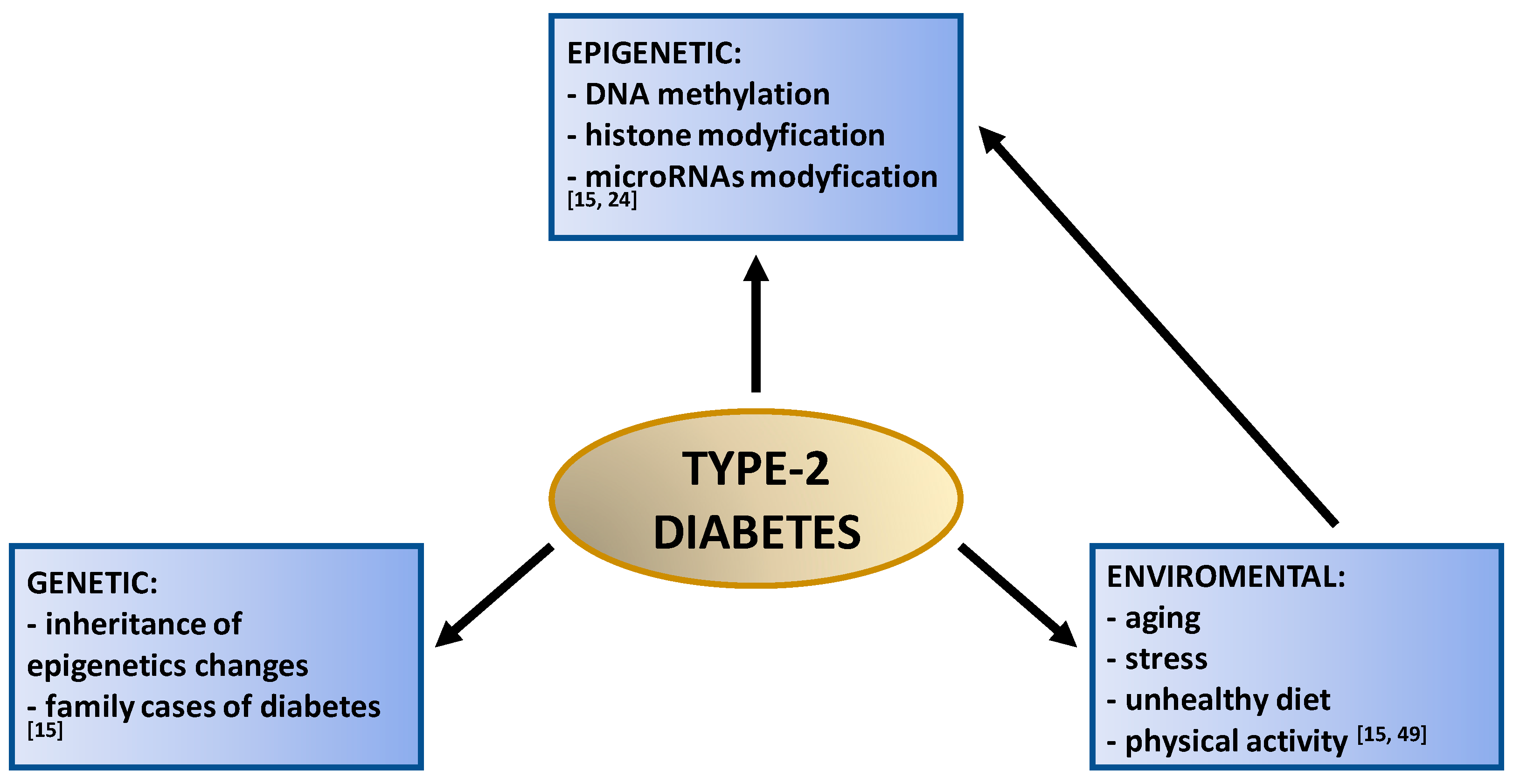
Type-2 Diabetes
7. Cyclitols in Treatment
7.1. Pinitol
7.2. Myo-Inositol and D-Chiro-Inositol
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| DCI | D-chiro-inositol |
| DP | D-pinitol |
| FFA | Free fatty acids |
| GDM | Gestational diabetes mellitus |
| GLUT | Glucose transporter |
| HDL | High-density lipoprotein |
| IPG | Inositol phosphoglycans |
| LDL | Low-density lipoprotein |
| MI | Myo-inositol |
| PCOS | Polycystic ovary syndrome |
| STZ | Streptozotocin-induced diabetes |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| MI-IPG | Myo-inositol phosphor glycan |
References
- Osowski, A.; Kasparek, A.; Wieczorek, Z.; Amarowicz, R.; Szabelski, M. Evaluation of the characteristics of some plant polyphenols as molecules intercepting mitoxantrone. Food Chem. 2017, 227, 142–148. [Google Scholar] [CrossRef]
- Carlomagno, G.; Unfer, V. Inositol safety: Clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 931–936. [Google Scholar]
- Pak, Y.; Hong, Y.; Kim, S.; Piccariello, T.; Farse, R.; Larner, J. In vivo chiro-inositol metabolism in the rat: A defect in chiro-inositol synthesis from myo-inositol and an increased incorporation of chiro-[3H] inositol into phospolipid in the Goto-Kakizaki (G.K.) rat. Mol. Cells 1998, 8, 301–309. [Google Scholar]
- Michell, R. Inositol and its derivatives: Their evolution and functions. Adv. Enzym. Regul. 2011, 51, 84–90. [Google Scholar] [CrossRef]
- Kornienko, A.; d’Alarcao, M. Synthesis of cyclitols via ring-closing metathesis. Tetrahedron Asymmetry 1999, 10, 827–829. [Google Scholar] [CrossRef]
- Wassink, A.; Olijhoek, J.; Visseren, F. The metabolic syndrome: Metabolic changes with vascular consequences. Eur. J. Clin. Investig. 2007, 37, 8–17. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomango, G.; Rizzo, P.; Raffone, E.; Roseff, S. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 452–457. [Google Scholar]
- Facchinetti, F.; Bizzarri, M.; Benvenga, S.; D’Anna, R.; Lanzone, A.; Soulage, C.; Di Renzo, G.; Hod, M.; Cavalli, P.; Chiu, T.; et al. Results from the international consensus conference on Myo-inositol and D-chiro-inositol in obstetrics and gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 72–76. [Google Scholar] [CrossRef]
- Martin, K.; Mani, M.; Mani, A. New targets to treat obesity and the metabolic syndrome. Eur. J. Pharm. 2015, 763, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Unfer, V.; Facchinetti, F.; Orru, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef]
- Rastegar, S.; Soltani, S.; Roohipoor, A.; Ebrahimi, E. Study of plants with D-chiro-inositol and its derivatives on diabetes. Int. J. Pharmacol. 2017, 4, 43–53. [Google Scholar]
- Croze, M.; Soulage, C. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochemie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Song, F.; Su, H.; Yang, N.; Zhu, L.; Cheng, J.; Wang, L.; Cheng, X. Myo-Inositol content determined by Myo-inositol biosynthesis and oxidation in blueberry fruit. Food Chem. 2010, 210, 381–387. [Google Scholar] [CrossRef]
- Capasso, I.; Esposito, E.; Maurea, N.; Montella, M.; Crispo, A.; De Laurentiis, M.; D’Aiuto, M.; Frasci, G.; Botti, G.; Grimaldi, M.; et al. Combination of inositol and alpha lipoic acid in metabolic syndrome-affected women: A randomized placebo-controlled trial. Trials 2013, 14, 273. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to Type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Ostlund, R.; McGill, J.; Herskowitz, I.; Kipnis, D.; Santiago, J.; Sherman, W. D-chiro-Inositol metabolism in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1993, 90, 9988–9992. [Google Scholar] [CrossRef]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Myoinositol and D-chiro inositol in improving insulin resistance in obese male children: Preliminary data. Int. J. Endocrinol. 2016, 206, 8720342. [Google Scholar] [CrossRef]
- Merchant, A.; Arndt, S.; Rowell, D.; Posch, S.; Callister, A.; Tausz, M.; Adams, M. Seasonal changes in carbohydrates, cyclitols, and water relations of 3 field grown Eucalyptus species from contrasting taxonomy on a common site. Ann. For. Sci. 2010, 67, 104. [Google Scholar] [CrossRef]
- Nordio, M.; Proietti, E. The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inostiol supplementation alone. Eur. Rev. Med. Pharmcol. Sci. 2012, 16, 575–581. [Google Scholar]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarii, M. Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar] [CrossRef]
- Davis, A.; Christiansen, M.; Horowitz, J.; Klein, S.; Hellerstein, M.; Ostlund, R. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef]
- Cogram, P.; Tesh, S.; Tesh, J.; Wade, A.; Allan, G.; Green, N.; Copp, A. D-chiro-inositol is more effective than myo-inositol in preventing folate-resistant mouse neural tube defects. Hum. Reprod. 2002, 17, 2451–2458. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Haley, A.; Gonzales, M.; Tarumi, T.; Miles, S.; Goudarzi, K.; Tanaka, H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab. Brain Dis. 2010, 25, 397–405. [Google Scholar] [CrossRef]
- Karla, S.; Karla, B. Inositols in midlife. J. Mid Life Health 2018, 9, 36–38. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M. Type-2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Lahuta, L.; Placek, W.; Górecki, R. The potential benefits of plant cyclitols in the treatment of psoriasis. Pol. Ann. Med. 2018, 25, 166–171. [Google Scholar] [CrossRef]
- Dinicola, S.; Chiu, T.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rational of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharmacol. 2014, 20, 1079–1092. [Google Scholar] [CrossRef]
- Ferrari, F.; Facchinetti, F.; Ontiveros, A.; Roberts, R.; Saade, M.; Blackwell, S.; Sibai, B.; Refuerzo, J.; Longo, M. The effect of combined inositol supplementation on maternal metabolic profile in pregnancies complicated by metabolic syndrome and obesity. Am. J. Obestetrics Gynecol. 2016, 215, 503. [Google Scholar] [CrossRef]
- Manheimer, E.; Zuuren, E.; Federowicz, Z.; Pijl, H. Paleolithic nutrition for metabolic syndrome: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 922–932. [Google Scholar] [CrossRef]
- Larner, J.; Brautigan, D.; Thorner, M. D-Chiro-Inositol glycans in insulin signalling and insulin resistance. Mol. Med. 2010, 16, 543–551. [Google Scholar] [CrossRef]
- Altaf, Q.; Barnett, A.; Tahrani, A. Novel therapeutics for type-2 diabetes: Insulin resistance. Diabetes Obes. Metab. 2015, 17, 319–334. [Google Scholar] [CrossRef]
- Tianrong, Z.; Hongxiang, L. Synthesis of azolenucleoside analogues of D-pinitol as potential antitumor agents. Carbohydr. Res. 2007, 342, 865–869. [Google Scholar]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Haribabu, L.; Nishigaki, I.; Balasubramanian, M. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-kappa B. Asian Pac. J. Cancer Prev. 2014, 15, 1757–1762. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, R.; Nandakumar, N.; Balasubramanian, M.; Nishigaki, I. Free radical scavenging and antioxidant activity of D-pinitol against 7, 12–dimethylbenz(a) anthacene induced breast cancer in spraguedawley rats. Asian Pac. J. Trop. Dis. 2014, 4, 584–590. [Google Scholar] [CrossRef]
- Ghosh, A.; Sen, D.; Bhattacharya, S. A new alkaloid isolated from Abieswebbiana leaf. Pharmacogn. Res. 2010, 2, 186–189. [Google Scholar]
- Lopez-Sanchez, J.; Moreno, D.; Garcia-Viguera, C. D-pinitol, a highly valuable product from carob pods: Health-promoting effects and metabolic pathways of this natural super-food ingredient and its derivatives. Aims Agric. Food 2018, 3, 41–63. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subramanian, S. D-pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen. Physiol. Biophys. 2009, 28, 233–241. [Google Scholar] [CrossRef]
- Endringer, D.; Oliveira, O.; Braga, F. In vitro and in silico inhibition of angiotensyn-converting enzyme by carbohydrates and cyclitols. Chem. Pap. 2014, 68, 37–45. [Google Scholar] [CrossRef]
- Alberti, G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new world-wide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ferder, L.; Inserra, F.; Martinez-Maldonado, M. Inflammation and the metabolic syndrome: Role of angiotensin II and oxidative stress. Curr. Hypertens. Rep. 2006, 8, 191–198. [Google Scholar] [CrossRef]
- Reza, M.; Khosrow, A. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 2009, 42, 1331–1346. [Google Scholar]
- Al-Suod, H.; Ligor, M.; Ratiu, I.; Rafińska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Olokoba, A.; Obateru, O.; Olokoba, L. Type-2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Oszukowski, P.; Jakimiuk, A.; Spaczyński, M.; Szamatowicz, J.; Karowicz-Bilińska, A.; Nowak-Markwitz, E.; Putowski, L.; Isaat, T. Stanowisko zespołu ekspertów polskiego towarzystwa ginekologicznego dotyczące stosowania preparatów zawierających myo-inozytol przez pacjentki z zespołem policystycznych jajników (PCOS). Ginekol. Pol. 2014, 2, 158–160. [Google Scholar]
- Kaisaki, P.; Delepine, M.; Woon, P.; Sebag-Montefiore, L.; Wilder, S.; Menzel, S.; Vionnet, N.; Marion, E.; Riveline, J.; Charpentier, G.; et al. Polymorphisms in type II SH2 domain—Containing inositol 5-phospatase (INPPL1, SHIP2) are associated with physiological abnormalities of the metabolic syndrome. Diabetes 2004, 53, 1900–1904. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, N.; Hu, Z.; Wang, L.; Liu, X.; Zhang, S. Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des. Dev. Ther. 2017, 11, 313–324. [Google Scholar] [CrossRef]
- Lalitha, P.; Sripathi, S. In silico ligand—Receptor docking of few cyclitols for type II diabetes using hex. Pharma Sci. Monit. Int. J. Pharm. Sci. 2011, 2, 32–41. [Google Scholar]
- Owczarczyk-Saczonek, A.; Lahuta, L.; Ligor, M.; Placek, W.; Górecki, R.; Buszewski, B. The healing-promoting properties of selected cyclitols—A review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef]
- Saleem, F.; Rizvi, A. New therapeutic approaches in obesity and metabolic syndrome associated with polycystic ovary syndrome. Cureus 2017, 9, e1844. [Google Scholar] [CrossRef]
- Rendle, P.; Kassibawi, F.; Johnston, K.; Hart, J.; Cameron, S.; Falshaw, A.; Painter, G.; Loomes, K. Synthesis and biological activities of D-chiro-inositol analogues with insulin-like actions. Eur. J. Med. Chem. 2016, 122, 442–451. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, K.; Kim, J.; Seo, Y.; Ha, B.; Kho, J.; Shin, Y.; Chung, C. Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type-2 diabetes. Diabetes Res. Clin. Pract. 2007, 77, 247–251. [Google Scholar] [CrossRef]
- Chang, H.; Choong, B.; Phillips, A.; Loomes, K. The diabetic rat kidney mediates inosituria and selective urinary partitioning of D-chiro-inositol. Exp. Biol. Med. 2015, 240, 8–14. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wang, T.; Wu, T.; Ai, R.; Zhang, Z. Hypoglycaemic effect of D-chiro-inositol in type-2 diabetes mellitus rats through the PI3K/Aktsignalling pathway. Mol. Cell. Endocrinol. 2016, 433, 26–34. [Google Scholar] [CrossRef]
- Tosti, C.; Cappelli, V.; De Leo, V. A new oral formulation based on D-chiro-inositol/monacolin K/bergamot extract/methylfolate and Vitamin K2 in prevention and treatment of metabolic syndrome in perimenopausal women with a BMI > 25kg/m2. J. Metab. Syndr. 2016, 5, 207. [Google Scholar] [CrossRef]
- Bates, S.; Jones, R.; Bailey, C. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948. [Google Scholar] [CrossRef] [Green Version]
- Nestler, J.; Jakubowicz, D.; Reamer, P.; Gunn, R.; Allan, G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef]
- Moreira, L.; Silva, J.; Silva, G.; Lemos, V.; Cortes, S. Activation of eNos by D-pinitol induces an endothelium-dependent vasodilation in mouse mesenteric artery. Front. Pharmacol. 2018, 9, 528. [Google Scholar] [CrossRef]
- Plows, J.; Budin, F.; Andersson, R.; Mills, V.; Mace, K.; Davidage, S.; Vickers, M.; Baker, P.; Silva-Zolezzi, I.; Stanley, J. The effects of myo-inositol and B and D Vitamin supplementation in the db/+ mouse model of gestational diabetes mellitus. Nutrients 2017, 9, 141. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Pande, M.; Seal, A.; Mishra, S.; Dasgupta, A.; Sengupta, M.; Dastider, R. The effects of combined therapy of myo-inositol and D-chiro-inositol in reduction of the individual components of metabolic syndrome in overweight PCOS patients compared to myo-inositol supplementation alone: A prospective randomised controlled trial. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 2939–2943. [Google Scholar] [CrossRef]
- Croze, M.; Geloen, A.; Soulage, C. Abnormalities in myo-inositol metabolism associated with type-2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.C.; Kang, M.; Lee, M.; Kim, J.; Cha, I. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: A randomized controlled study. Eur. J. Clin. Nutr. 2005, 59, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Singh, B.; Choudhary, S.; Kumar, A. Animal models on insulin resistance: A review. Pharmacol. Rep. 2016, 68, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Galletta, M.; Grasso, S.; Vaiarelli, A.; Roseff, S. Bye-bye Chiro-inositol–Myo-inositol: True progress in the treatment of polycystic ovary syndrome and ovulation induction. Eur. Rev. Med Pharmacol. Sci. 2011, 15, 1212–1214. [Google Scholar]
- Dang, N.; Mukai, R.; Yoshida, K.; Ashida, H. D-Pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of myo-inositol and d-chiro-inositol treatment in type-2 diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef]
- Maeba, R.; Hara, H.; Ishikawa, H.; Hayashi, S.; Yoshimura, N.; Kusano, J.; Taboka, Y.; Yasuda, D.; Okazaki, T.; Kinoshita, M.; et al. Myo-inositol treatment increases serum plasmalogens and decreases small dense LDL, particularly in hyperlipidemic subjects with metabolic syndrome. J. Nutr. Sci. Vitam. 2008, 54, 196–202. [Google Scholar] [CrossRef]
- Lien, L.; Guyton, J. Metabolic syndrome. Dermatol. Ther. 2008, 21, 362–375. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Lee, J.; Chung, S.; Park, S.; Kang, H.; Yang, E.; Cho, H.; Lee, K. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 2004, 27, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Risérus, U.; Arner, P.; Brismar, K.; Vessby, B. Treatment with dietary trans10cis12 conjugated linoleic acid causes Isomerspecific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002, 25, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seol, I.; Son, C. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Chang, H.; Chao, H.; Walker, C.; Choong, S.; Phillips, A.; Loomes, K. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Ren. Physiol. 2015, 309, 755–763. [Google Scholar] [CrossRef] [PubMed]


| No. | Authors | Cyclitol | Object/Dose | Major In Vivo & In Vitro Effects |
|---|---|---|---|---|
| 1. | Gao et al. (2016) [55] | DCI | Rats/group I: 30 mg/kg; group II: 60 mg/kg | - reduction of blood glucose level; - significant reduction of insulin level in serum - regulation of glycogen synthase and glucose transporter type-4; - increasing glucose transporter type-4 expression in skeletal muscles; - regulation of insulin-mediated glucose uptake; |
| 2. | Tosti et al. (2016) [56] | DCI | Women/ 100 mg/for each test subject | - BMI reduction; - reduction of blood sugar and lipid values; - reduction of total cholesterol; - reduction of triglyceride values; |
| 3. | Rengarajan et al. (2014) [34] | DP | MCF-7 cell line/20, 40, 60, 80, 100, 120 µM | - significant inhibition of MCF-7 cell proliferation in a concentration-dependent manner; - an increase in p53 and Bax and a decrease in Bcl-2 and NF-κBexpression; |
| 4. | Bates at al. (2000) [57] | DP | Obese-diabeticob/ob mice/100 mg/kg | - a decrease in plasma glucose level; |
| 5. | Unfer et al. (2011) [7] | MI; DCI | Women/ 2 g/for each test subject | - reduction of immature oocytes in the myo-inositol treatment group compared to the D-chiro-inositol treatment group |
| 6. | Moreira et al. (2018) [59] | DP | C57BL/6 mice/ 10 mg/kg | - an increase in the concentration of nitrite in blood, which was inhibited by L-NAME and calmidazolium; - reduction of systolic blood pressure; |
| 7. | Plow et al. (2017) [60] | MI | LepRdb/+ (db/+) mice/10 g/kg | - reduction of weight and fat storage; - reduction of inflammatory marker expression in adipose tissue; - a reduction in the hyperleptinemia observed in db/+ mice; - increasing insulin sensitivity and glucose uptake; |
| 8. | Nordio et al. (2012) [19] | MI; DCI | Women/550 mg of MI plus 13,8 mg of DCI twice a day | - improvement of the metabolic parameters; |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonowski, T.; Osowski, A.; Lahuta, L.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients 2019, 11, 2314. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102314
Antonowski T, Osowski A, Lahuta L, Górecki R, Rynkiewicz A, Wojtkiewicz J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients. 2019; 11(10):2314. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102314
Chicago/Turabian StyleAntonowski, Tomasz, Adam Osowski, Lesław Lahuta, Ryszard Górecki, Andrzej Rynkiewicz, and Joanna Wojtkiewicz. 2019. "Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes" Nutrients 11, no. 10: 2314. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11102314





