Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Interventions and Tissue Collection
2.2. Bacterial Genomic DNA Extraction
2.3. Bioinformatics Sequencing and Analysis
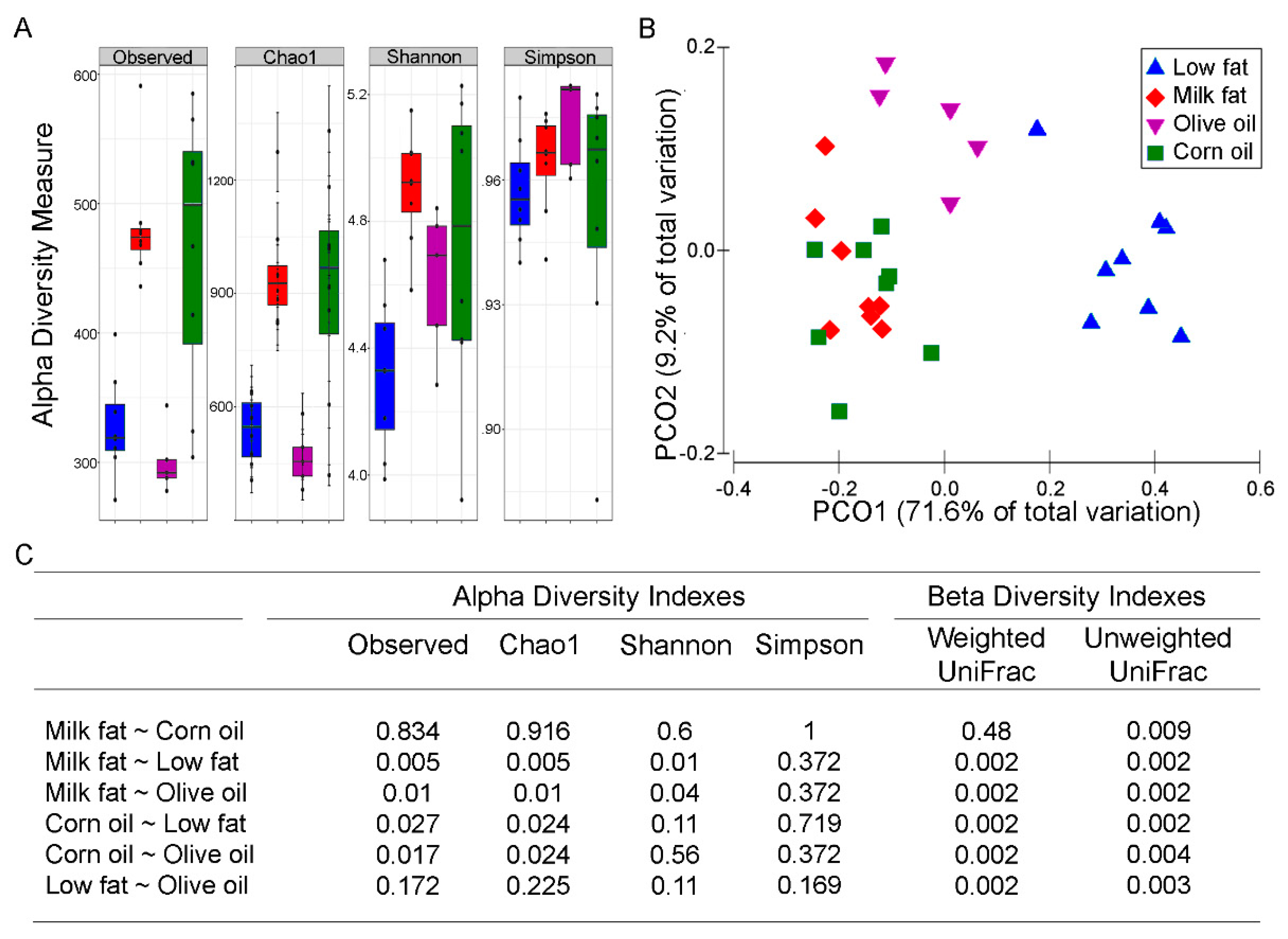
2.4. Alpha and Beta Diversity Analysis
2.5. Abundance Analysis
2.6. Amplicon Sequencing Prediction Analysis
2.7. Short-Chain Fatty Acid Analysis
2.8. Protein Extraction
2.9. Protein Digestion, Itraq Labeling and LC-MS/MS Analysis
2.10. Protein Data Processing and Sequence Database Searching
2.11. Ingenuity Pathways Analysis for Mucosal Host Proteins
2.12. Statistical Analysis
2.13. Data Availability
2.14. Ethical Considerations
3. Results
3.1. Dietary Lipid Type Affects Gut Microbial Diversity
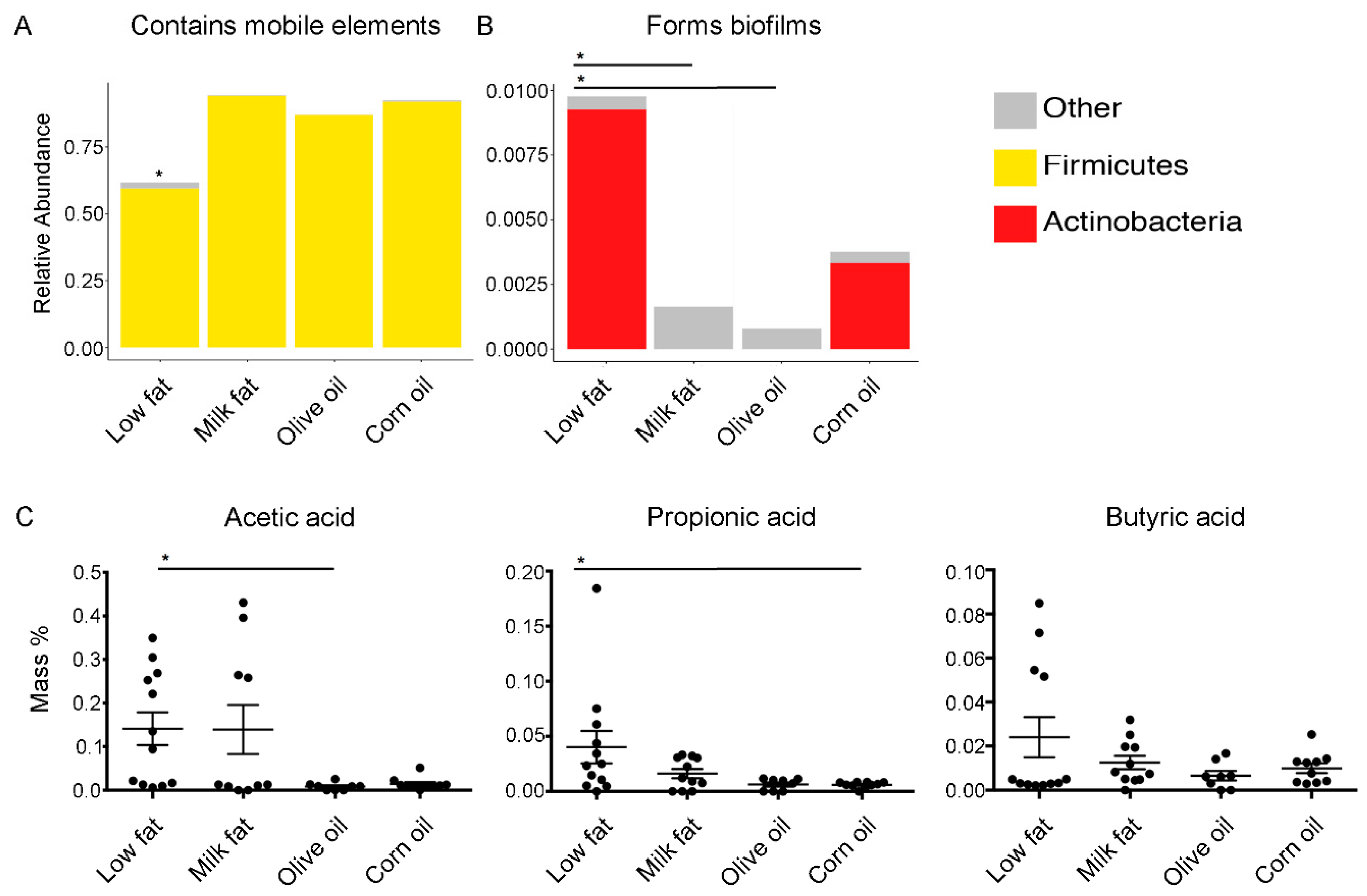
3.2. Dietary Lipid Type Confers Core Functionality to Each Microbial Community
3.3. Dietary Lipids Alter Microbial and Host Proteins in the Colon
3.3.1. High-Fat Diets Associated with Decreased Death Receptor Signaling and Apoptosis and tRNA Charging
3.3.2. Corn Oil Diets Show Responses Indicative of Increased Energy Requirements and Oxidative Stress, and Decreased Barrier Function
3.3.3. Milk Fat Diet is Associated with Increased Inflammation and Compensating Restitution
3.3.4. Olive Oil Consumption Was Associated with Increased Cytoskeletal Dynamics
3.4. Microbial Taxa Associate with Host Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vernocchi, P.; Vannini, L.; Gottardi, D.; Del Chierico, F.; Serrazanetti, D.I.; Ndagijimana, M.; Guerzoni, M.E. Integration of datasets from different analytical techniques to assess the impact of nutrition on human metabolome. Front. Cell. Infect. Microbiol. 2012, 2, 156. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Estaki, M.; Gibson, D.L. Clinical consequences of diet-induced dysbiosis. Ann. Nutr. Metab. 2013, 63, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Gordon, J.I. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, S.M.; Wang, M.; Li, M.; Friedberg, I.; Schwartz, S.L.; Chapkin, R.S. Host-microbe interactions in the neonatal intestine: Role of human milk oligosaccharides. Adv. Nutr. (Bethesda) 2012, 3, 450S–455S. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Camuesco, D.; Galvez, J.; Nieto, A.; Comalada, M.; Rodriguez-Cabezas, M.E.; Concha, A.; Xaus, J.; Zarzuelo, A. Dietary olive oil supplemented with fish oil, rich in epa and dha (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with dss-induced colitis. J. Nutr. 2005, 135, 687–694. [Google Scholar] [CrossRef] [PubMed]
- DeCoffe, D.; Quin, C.; Gill, S.K.; Tasnim, N.; Brown, K.; Godovannyi, A.; Dai, C.; Abulizi, N.; Chan, Y.K.; Ghosh, S.; et al. Dietary lipid type, rather than total number of calories, alters outcomes of enteric infection in mice. J. Infect. Dis. 2016, 213, 1846–1856. [Google Scholar] [CrossRef]
- Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; Berglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a european prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar]
- Ghosh, S.; Molcan, E.; DeCoffe, D.; Dai, C.; Gibson, D.L. Diets rich in n-6 pufa induce intestinal microbial dysbiosis in aged mice. Br. J. Nutr. 2013, 110, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs lps dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef]
- Opstelten, J.L.; Leenders, M.; Dik, V.K.; Chan, S.S.; van Schaik, F.D.; Khaw, K.T.; Luben, R.; Hallmans, G.; Karling, P.; Lindgren, S.; et al. Dairy products, dietary calcium, and risk of inflammatory bowel disease: Results from a european prospective cohort investigation. Inflamm. Bowel Dis. 2016, 22, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.a.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, A.; Ramakrishna, B.S.; Pulimood, A.B.; Patra, S.; Murthy, S. Increased permeability in dextran sulphate colitis in rats: Time course of development and effect of butyrate. Scand. J. Gastroenterol. 2000, 35, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.H.; Albert, M.J.; Hamidur Rahman, A.S.; Moyenul Isalm, M.; Nasirul Islam, K.M.; Alam, K. Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J. Infect Dis. 1999, 179, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A.; et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in nod mice. ISME J. 2016, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Expected Errors Predicted by Phred (q) Scores. Available online: https://www.drive5.com/usearch/manual/exp_errs.html (accessed on 15 February 2019).
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. Pynast: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16s rrna marker gene sequences. Nat Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. Unifrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecology 2008, 26, 32–46. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. Kegg for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Ward, T.; Larson, J.; Meulemanns, J.; Hillmann, B.; Lynch, J.; Sidiropoulos, D.; Spear, J.; Caporaso, G.; Blekman, R.; Knight, R.; et al. Bugbase predicts organism level microbiome phenotypes. bioRxiv 2017. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, A.; Meng, K.; Chang, W.; Bai, Y.; Li, K.; Cai, H.; Liu, G.; Yao, B. Proteome changes in the intestinal mucosa of broiler (gallus gallus) activated by probiotic enterococcus faecium. J. Proteom. 2013, 91, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Chai, Y.L.; Tan, J.M.; Lee, J.H.; Francis, P.T.; Chen, C.P.; Sze, S.K.; Lai, M.K.P. An itraq-based proteomic analysis reveals dysregulation of neocortical synaptopodin in lewy body dementias. Mol. Brain 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Qian, J.; Chong, R.; Kalaria, R.N.; Francis, P.; Lai, M.K.; Chen, C.P.; Sze, S.K. Novel pathophysiological markers are revealed by itraq-based quantitative clinical proteomics approach in vascular dementia. J. Proteom. 2014, 99, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Sze, S.K. Data for itraq profiling of micro-vesicular plasma specimens: In search of potential prognostic circulatory biomarkers for lacunar infarction. Data Brief 2015, 4, 510–517. [Google Scholar] [CrossRef]
- Yang, W.; Woltjer, R.L.; Sokal, I.; Pan, C.; Wang, Y.; Brodey, M.; Peskind, E.R.; Leverenz, J.B.; Zhang, J.; Perl, D.P.; et al. Quantitative proteomics identifies surfactant-resistant alpha-synuclein in cerebral cortex of parkinsonism-dementia complex of guam but not alzheimer’s disease or progressive supranuclear palsy. Am. J. Pathol. 2007, 171, 993–1002. [Google Scholar] [CrossRef]
- Randall, D.R.; Park, P.S.; Chau, J.K. Identification of altered protein abundances in cholesteatoma matrix via mass spectrometry-based proteomic analysis. J. Otolaryngol Head Neck Surg. 2015, 44, 50. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Nesvizhskii, A.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Mistry, D. Ipa: Maximizing the Biological Interpretation of Gene Transcript & Protein Expression Data with IPA, 2018. Available online: https://bioinformatics.rockefeller.edu/wp-content/uploads/IPA-Training-Dev.pdf (accessed on 15 February 2019).
- Overgaard, A.J.; Thingholm, T.E.; Larsen, M.R.; Tarnow, L.; Rossing, P.; McGuire, J.N.; Pociot, F. Quantitative itraq-based proteomic identification of candidate biomarkers for diabetic nephropathy in plasma of type 1 diabetic patients. Clin. Proteom. 2010, 6, 105–114. [Google Scholar] [CrossRef]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, X.; Huang, H. Sampling strategies for three-dimensional spatial community structures in ibd microbiota research. Front. Cell. Infect. Microbiol. 2017, 7, 51. [Google Scholar] [CrossRef]
- Tang, M.S.; Poles, J.; Leung, J.M.; Wolff, M.J.; Davenport, M.; Lee, S.C.; Lim, Y.A.; Chua, K.H.; Loke, P.; Cho, I. Inferred metagenomic comparison of mucosal and fecal microbiota from individuals undergoing routine screening colonoscopy reveals similar differences observed during active inflammation. Gut Microbes 2015, 6, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Baumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, I.L.; Yilmaz, S.; Huang, K.; Xu, L.; Jupiter, S.D.; Jenkins, A.P.; Naisilisili, W.; Tamminen, M.; Smillie, C.S.; Wortman, J.R.; et al. Mobile genes in the human microbiome are structured from global to individual scales. Nature 2016, 535, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodaran, S.; Wood, T.D.; Nagarajan, P.; Rabin, R.A. Evaluating peptide mass fingerprinting-based protein identification. Genom. Proteom. Bioinform. 2007, 5, 152–157. [Google Scholar] [CrossRef]
- IPA. Ingenuity Pathways Analysis Software. Available online: http://www.ingenuity.com (accessed on 15 February 2019).
- Sayedyahossein, S.; Rudkouskaya, A.; Leclerc, V.; Dagnino, L. Integrin-linked kinase is indispensable for keratinocyte differentiation and epidermal barrier function. J. Investig. Dermatol 2016, 136, 425–435. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins link inflammation and metabolism. J. Immunol. Res. 2016, 2016, 8167273. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.L.; Goncalves, L.M.; de Vasconcellos, A.A.; da Silva, W.J.; Cury, J.A.; Del Bel Cury, A.A. Dietary carbohydrates modulate candida albicans biofilm development on the denture surface. PLoS ONE 2013, 8, e64645. [Google Scholar] [CrossRef]
- O’Donnell, M.M.; O’Toole, P.W.; Ross, R.P. Catabolic flexibility of mammalian-associated lactobacilli. Microb. Cell Fact. 2013, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petschow, B.; Dore, J.; Hibberd, P.; Dinan, T.; Reid, G.; Blaser, M.; Cani, P.D.; Degnan, F.H.; Foster, J.; Gibson, G.; et al. Probiotics, prebiotics, and the host microbiome: The science of translation. Ann. N. Y. Acad. Sci. 2013, 1306, 1–17. [Google Scholar] [CrossRef]
- Ghosh, S.; Sulistyoningrum, D.C.; Glier, M.B.; Verchere, C.B.; Devlin, A.M. Altered glutathione homeostasis in heart augments cardiac lipotoxicity associated with diet-induced obesity in mice. J. Biol. Chem. 2011, 286, 42483–42493. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Botta, A.; Pither, J.; Dai, C.; Gibson, W.T.; Ghosh, S. A high-fat diet rich in corn oil reduces spontaneous locomotor activity and induces insulin resistance in mice. J. Nutr. Biochem. 2015, 26, 319–326. [Google Scholar] [CrossRef]
- Simopoulos, A.; Cleland, L.G. Omega-6/omega-3 essential fatty acid ratio: The scientific evidence. World Rev. Nutr. Diet. 2003, 92, 1–194. [Google Scholar]
- Hart, A.R.; Luben, R.; Olsen, A.; Tjonneland, A.; Linseisen, J.; Nagel, G.; Berglund, G.; Lindgren, S.; Grip, O.; Key, T.; et al. Diet in the aetiology of ulcerative colitis: A european prospective cohort study. Digestion 2008, 77, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef]
- Innis, S.M.; Dai, C.; Wu, X.; Buchan, A.M.; Jacobson, K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1376–G1385. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.M.; Grimble, R.F. Effect of dietary linoleate content on the metabolic response of rats to escherichia coli endotoxin. Clin. Sci. (Lond) 1987, 72, 383–385. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Ishizuka, I.; Araki, Y.; Sasaki, M.; Koyama, S.; Fujiyama, Y. N-3 fatty acid-rich diet prevents early response of interleukin-6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int. J. Mol. Med. 2003, 12, 721–725. [Google Scholar] [CrossRef]
- Hekmatdoost, A.; Wu, X.; Morampudi, V.; Innis, S.M.; Jacobson, K. Dietary oils modify the host immune response and colonic tissue damage following citrobacter rodentium infection in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G917–G928. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Myung, S.J.; Do, M.Y.; Ryu, Y.M.; Kim, M.J.; Do, E.J.; Park, S.; Yoon, S.M.; Ye, B.D.; Byeon, J.S.; et al. Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J. Gastroenterol. Hepatol. 2010, 25, 1785–1794. [Google Scholar] [CrossRef]
- Ramos, H.J.; Gale, M., Jr. Rig-i like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr. Opin. Virol. 2011, 1, 167–176. [Google Scholar] [CrossRef]
- Mazumdar, B.; Kim, H.; Meyer, K.; Bose, S.K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Hepatitis c virus proteins inhibit c3 complement production. J. Virol. 2012, 86, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Hill-Batorski, L.; Halfmann, P.; Marzi, A.; Lopes, T.J.; Neumann, G.; Feldmann, H.; Kawaoka, Y. Loss of interleukin 1 receptor antagonist enhances susceptibility to ebola virus infection. J. Infect. Dis. 2015, 212, S329–S335. [Google Scholar] [CrossRef] [PubMed]



| Pathway | Symbol | Gene Name | Low Fat | Milk Fat | Olive Oil | Corn Oil |
|---|---|---|---|---|---|---|
| High fat | ||||||
| Death Receptor | ACIN1 | apoptotic chromatin condensation inducer 1 | 0.4 | −0.2 | 0 | −0.3 |
| signaling | CYCS | cytochrome c, somatic | 0.7 | 0.3 | −0.2 | −0.2 |
| HSPB1 | heat shock protein family B (small) member 1 | −0.5 | 0.5 | 0.1 | −0.2 | |
| LMNA | lamin A/C | 0.6 | −0.2 | −0.1 | −0.4 | |
| SPTAN1 | spectrin alpha, non-erythrocytic 1 | 0.6 | −0.1 | −0.1 | −0.2 | |
| Apoptosis | ACIN1 | apoptotic chromatin condensation inducer 1 | 0.4 | −0.2 | 0 | −0.3 |
| CAPN1 | calpain 1 | 0.8 | 0 | −0.1 | −0.1 | |
| CYCS | cytochrome c, somatic | 0.7 | 0.3 | −0.2 | −0.2 | |
| LMNA | lamin A/C | 0.6 | −0.2 | −0.1 | −0.4 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| SPTAN1 | spectrin alpha, non-erythrocytic 1 | 0.6 | −0.1 | −0.1 | −0.2 | |
| IL1RN | Interleukin-1 receptor antagonist protein | −1 | 0.4 | -0.1 | 0.7 | |
| tRNA charging | EPRS | glutamyl-prolyl-tRNA synthetase | 0.8 | −0.1 | −0.2 | 0 |
| FARSB | phenylalanyl-tRNA synthetase beta subunit | 0.7 | 0 | −0.2 | −0.1 | |
| KARS | lysyl-tRNA synthetase | 0.8 | −0.2 | −0.3 | −0.2 | |
| NARS | asparaginyl-tRNA synthetase | 0.9 | −0.3 | −0.5 | −0.4 | |
| RARS | arginyl-tRNA synthetase | 0.7 | −0.1 | −0.2 | 0 | |
| TARS | threonyl-tRNA synthetase | 0.8 | 0 | −0.2 | −0.1 | |
| VARS | valyl-tRNA synthetase | 0.4 | 0 | −0.1 | −0.2 | |
| YARS | tyrosyl-tRNA synthetase | 0.5 | −0.4 | −0.3 | −0.2 | |
| PPARa/RXRa | ACOX1 | acyl-CoA oxidase 1 | 0.5 | 0 | −0.3 | −0.4 |
| Activation | APOA1 | apolipoprotein A1 | −0.7 | 0.2 | −0.1 | 0.5 |
| CYP2C18 | cytochrome P450 family 2 subfamily C member 18 | 0.3 | −0.4 | 0.4 | −1.7 | |
| FASN | fatty acid synthase | 0 | 0 | 0 | 0.4 | |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | 1.3 | −0.6 | −0.4 | −0.3 | |
| HSP90B1 | heat shock protein 90 beta family member 1 | 0.2 | −0.4 | −0.3 | −0.1 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| PDIA3 | protein disulfide isomerase family A member 3 | −0.5 | 0 | 0 | 0.1 | |
| Corn oil | ||||||
| Glycolysis I | ALDOB | aldolase, fructose-bisphosphate B | 1.4 | −0.5 | −0.6 | −0.5 |
| ENO1 | enolase 1 | −0.5 | 0.2 | 0.1 | 0.3 | |
| FBP2 | fructose-bisphosphatase 2 | 0.2 | −0.2 | −0.2 | 0.1 | |
| TPI1 | triosephosphate isomerase 1 | −0.6 | 0 | 0 | 0.4 | |
| Oxidative | ATP5F1B | ATP synthase F1 subunit beta | −0.8 | 0.2 | 0 | 0.5 |
| phosphorylation | ATP5PB | ATP synthase peripheral stalk-membrane subunit b | 0.6 | −0.1 | −0.1 | −0.3 |
| ATP5PO | ATP synthase peripheral stalk subunit OSCP | −0.7 | 0.1 | 0.1 | 0.4 | |
| COX5A | cytochrome c oxidase subunit 5A | −0.9 | 0.3 | 0.2 | 0.6 | |
| CYCS | cytochrome c, somatic | 0.7 | 0.3 | −0.2 | −0.2 | |
| NDUFA9 | NADH:ubiquinone oxidoreductase subunit A9 | 0.8 | 0.2 | 0.2 | −0.1 | |
| NDUFS1 | NADH:ubiquinone oxidoreductase core subunit S1 | −0.4 | −0.2 | 0 | 0.4 | |
| NDUFS2 | NADH:ubiquinone oxidoreductase core subunit S2 | 0.6 | 0 | 0 | −0.1 | |
| NDUFS3 | NADH:ubiquinone oxidoreductase core subunit S3 | −0.8 | 0.1 | 0.1 | 0.3 | |
| NDUFV2 | NADH:ubiquinone oxidoreductase core subunit V2 | −0.3 | −0.1 | −0.1 | 0.3 | |
| UQCRB | ubiquinol-cytochrome c reductase binding protein | 0.2 | −0.3 | −0.3 | 0 | |
| UQCRC2 | ubiquinol-cytochrome c reductase core protein 2 | 0.3 | −0.1 | −0.1 | 0.1 | |
| NRF2-mediated | CBR1 | carbonyl reductase 1 | −0.4 | 0.1 | 0.1 | 0.2 |
| oxidative stress | CCT7 | chaperonin containing TCP1 subunit 7 | 0.5 | −0.2 | −0.2 | −0.3 |
| response | DNAJB11 | DnaJ heat shock protein family (Hsp40) member B11 | 0.7 | −0.2 | −0.4 | −0.5 |
| FTH1 | ferritin heavy chain 1 | −0.4 | 0.3 | 0 | 0.1 | |
| FTL | ferritin light chain | −0.2 | 0.2 | 0.2 | 0.3 | |
| GSR | glutathione-disulfide reductase | 0.6 | −0.1 | −0.2 | 0.1 | |
| GSTM3 | glutathione S-transferase mu 3 | 1.2 | 0.3 | 0.4 | 0.3 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| SOD1 | superoxide dismutase 1 | 0.6 | −0.1 | −0.2 | 0.2 | |
| USP14 | ubiquitin specific peptidase 14 | 0.6 | −0.2 | −0.2 | −0.1 | |
| CA3 | Carbonic anhydrase 3 | 0 | −0.1 | 0 | 0.7 | |
| ALDH2 | Aldehyde dehydrogenase | −0.6 | 0.2 | 0 | 0.6 | |
| Glutathione-mediated | ANPEP | alanyl aminopeptidase, membrane | 1.8 | −1.1 | −0.8 | −0.8 |
| detoxification | GGH | gamma-glutamyl hydrolase | 0.6 | 0.2 | −0.1 | 0.6 |
| Gsta4 | glutathione S-transferase, alpha 4 | 0.4 | 0 | 0 | −0.5 | |
| GSTM3 | glutathione S-transferase mu 3 | 1.2 | 0.3 | 0.4 | 0.3 | |
| GSTZ1 | glutathione S-transferase zeta 1 | −0.3 | −0.1 | 0.1 | 0.5 | |
| ILK signaling | ACTN1 | actinin alpha 1 | 0.3 | 0.1 | 0.1 | −0.3 |
| ACTN4 | actinin alpha 4 | 0.6 | −0.3 | −0.3 | −0.2 | |
| DSP | desmoplakin | 0.6 | −0.1 | 0 | −0.3 | |
| FLNA | filamin A | 0.4 | 0.2 | 0.3 | −0.4 | |
| FLNC | filamin C | 0.7 | 0.1 | 0.1 | −0.6 | |
| FN1 | fibronectin 1 | 0.7 | −0.1 | 0.1 | −0.8 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| MYH9 | myosin heavy chain 9 | 0.6 | −0.2 | −0.2 | −0.2 | |
| MYH11 | myosin heavy chain 11 | 0.7 | 0.2 | 0.4 | −0.6 | |
| MYH14 | myosin heavy chain 14 | 0.5 | −0.1 | −0.1 | −0.2 | |
| MYL9 | myosin light chain 9 | −0.5 | 0.4 | 0.6 | −0.2 | |
| PPP2R1A | protein phosphatase 2 scaffold subunit Alpha | −0.5 | 0.1 | 0.2 | 0.5 | |
| VCL | vinculin | 0.6 | −0.1 | 0.1 | −0.4 | |
| Epithelial integrity | Muc2 | mucin-2 | 0.2 | −0.1 | −0.2 | −0.6 |
| Cing | cingulin | 0.6 | −0.3 | −0.2 | −0.3 | |
| VEGF signaling | ACTN1 | actinin alpha 1 | 0.3 | 0.1 | 0.1 | −0.3 |
| ACTN4 | actinin alpha 4 | 0.6 | −0.3 | −0.3 | −0.2 | |
| EIF2S3 | eukaryotic translation initiation factor 2 subunit γ | 0.4 | −0.1 | −0.1 | −0.2 | |
| ELAVL1 | ELAV like RNA binding protein 1 | 0.7 | −0.1 | −0.1 | −0.1 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| VCL | vinculin | 0.6 | −0.1 | 0.1 | −0.4 | |
| Bleeding network | APOE | apolipoprotein E | −0.7 | 0.2 | −0.1 | 0.5 |
| CNN1 | cluster of calponin-1 | −0.1 | 0.5 | 0.5 | −0.2 | |
| FLNA | filamin-a | 0.4 | 0.2 | 0.3 | −0.4 | |
| MYH9 | cluster of myosin-9 | 0.6 | −0.2 | −0.2 | −0.2 | |
| PLEC | cluster of plectin | 0.4 | −0.1 | 0 | −0.5 | |
| IL1RN | interleukin-1 receptor antagonist protein | −1 | 0.4 | −0.1 | 0.7 | |
| Contractility of muscle network | ATP2A2 | sarcoplasmic/endoplasmic reticulum calcium ATPase | 0.5 | −0.2 | −0.1 | −0.4 |
| CKM | cluster of creatine kinase M-type | −0.3 | 0.2 | 0.3 | −0.3 | |
| DES | cluster of desmin | 0.6 | 0.4 | 0.3 | −0.5 | |
| MYH11 | cluster of myosin-11 | 0.7 | 0.2 | 0.4 | −0.6 | |
| MYH14 | myosin-14 | 0.5 | −0.1 | −0.1 | −0.2 | |
| VCL | vinculin | 0.6 | −0.1 | 0.1 | −0.4 | |
| Milk fat | ||||||
| Acute Phase Response | APOA1 | apolipoprotein A1 | −0.7 | 0.2 | −0.1 | 0.5 |
| C3 | complement C3 | 0.4 | −0.1 | −0.8 | −0.7 | |
| FN1 | fibronectin 1 | 0.7 | −0.1 | 0.1 | −0.8 | |
| FTL | ferritin light chain | −0.2 | 0.2 | 0.2 | 0.3 | |
| HP | haptoglobin | 0.8 | 0.2 | −1.6 | −1.3 | |
| IL1RN | interleukin 1 receptor antagonist | −1 | 0.4 | −0.1 | 0.7 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| SERPINA3 | serpin family A member 3 | −0.6 | 0.6 | −1.4 | −1.1 | |
| AAG1 | alpha-1 acid glycoprotein 1 | 0.8 | 0.4 | -0.8 | -0.6 | |
| Sirtuin signaling | ADAM10 | ADAM metallopeptidase domain 10 | 0.4 | −0.1 | −0.2 | 0 |
| APEX1 | apurinic/apyrimidinic endodeoxyribonuclease 1 | 0.7 | −0.1 | −0.2 | −0.3 | |
| ATP5F1B | ATP synthase F1 subunit beta | −0.8 | 0.2 | 0 | 0.5 | |
| ATP5PB | ATP synthase peripheral stalk-membrane subunit b | 0.6 | −0.1 | −0.1 | −0.3 | |
| CPS1 | carbamoyl-phosphate synthase 1 | 2.3 | −2 | −1.3 | −1.9 | |
| H1F0 | H1 histone family member 0 | −0.5 | 1.2 | 0.4 | −0.6 | |
| Hist1h1e | histone cluster 1, H1e | 0.3 | 1.1 | 0.1 | −0.6 | |
| HMGCS2 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 | −0.4 | 0 | −0.2 | −1.5 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| NAMPT | nicotinamide phosphoribosyltransferase | 0.6 | −0.1 | −0.2 | −0.3 | |
| NDUFA9 | NADH:ubiquinone oxidoreductase subunit A9 | 0.8 | 0.2 | 0.2 | −0.1 | |
| NDUFS1 | NADH:ubiquinone oxidoreductase core subunit S1 | −0.4 | −0.2 | 0 | 0.4 | |
| NDUFS2 | NADH:ubiquinone oxidoreductase core subunit S2 | 0.6 | 0 | 0 | −0.1 | |
| NDUFS3 | NADH:ubiquinone oxidoreductase core subunit S3 | −0.8 | 0.1 | 0.1 | 0.3 | |
| NDUFV2 | NADH:ubiquinone oxidoreductase core subunit V2 | −0.3 | −0.1 | −0.1 | 0.3 | |
| PDHA1 | pyruvate dehydrogenase E1 alpha 1 subunit | −0.3 | 0 | 0 | 0.1 | |
| SF3A1 | splicing factor 3a subunit 1 | 0.7 | 0 | 0.1 | −0.1 | |
| SLC25A5 | solute carrier family 25 member 5 | 0.9 | 0 | 0 | −0.2 | |
| SOD1 | superoxide dismutase 1 | 0.6 | −0.1 | −0.2 | 0.2 | |
| TIMM13 | translocase of inner mitochondrial membrane 13 | −0.2 | 0 | 0 | 0.3 | |
| UQCRC2 | ubiquinol-cytochrome c reductase core protein 2 | 0.3 | −0.1 | −0.1 | 0.1 | |
| VDAC1 | voltage dependent anion channel 1 | 0.1 | 0.3 | −0.1 | 0.2 | |
| Fatty acid B oxidation | ACAA2 | acetyl-CoA acyltransferase 2 | −0.4 | 0.1 | 0.2 | 0 |
| HADHA | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha | −0.1 | 0.2 | 0.1 | 0.2 | |
| HADHB | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta | −0.4 | 0.2 | 0.1 | 0.1 | |
| IVD | isovaleryl-CoA dehydrogenase | −0.6 | −0.1 | 0 | 0.2 | |
| Olive oil | ||||||
| Actin cytoskeleton | ACTN1 | actinin alpha 1 | 0.3 | 0.1 | 0.1 | −0.3 |
| signaling | ACTN4 | actinin alpha 4 | 0.6 | −0.3 | −0.3 | −0.2 |
| ARPC5 | actin related protein 2/3 complex subunit 5 | −0.4 | 0.2 | 0 | 0.4 | |
| FLNA | filamin A | 0.4 | 0.2 | 0.3 | −0.4 | |
| FN1 | fibronectin 1 | 0.7 | −0.1 | 0.1 | −0.8 | |
| IQGAP2 | IQ motif containing GTPase activating protein 2 | 0.7 | 0 | −0.1 | −0.2 | |
| MAPK1 | mitogen-activated protein kinase 1 | 0.2 | 0 | −0.2 | −0.1 | |
| MYH9 | myosin heavy chain 9 | 0.6 | −0.2 | −0.2 | −0.2 | |
| MYH11 | myosin heavy chain 11 | 0.7 | 0.2 | 0.4 | −0.6 | |
| MYH14 | myosin heavy chain 14 | 0.5 | −0.1 | −0.1 | −0.2 | |
| MYL9 | myosin light chain 9 | −0.5 | 0.4 | 0.6 | −0.2 | |
| VCL | vinculin | 0.6 | −0.1 | 0.1 | −0.4 | |
| Col6a3 | cluster of protein Col6a3 | −0.1 | -0.6 | 1.1 | −1.1 | |
| Tumorigenesis of | ACOX1 | acyl-coenzyme A oxidase 1 | 0.5 | 0 | −0.3 | −0.4 |
| tissue network | APOA1 | apolipoprotein a-1 | −0.7 | 0.2 | −0.1 | 0.5 |
| ATP2A2 | sarcoplasmic/endoplasmic reticulum calcium ATPase2 | 0.5 | −0.2 | −0.1 | −0.4 | |
| C3 | complement C3 | 0.4 | −0.1 | −0.8 | −0.7 | |
| HP | hippocalcin-like protein 1 | 0.8 | −0.2 | −0.2 | 0.2 | |
| IL1RN | interleukin-1 receptor antagonist protein | −1 | 0.4 | −0.1 | 0.7 | |
| MTTP | microsomal triglyceride transfer protein large subunit | 2.4 | −2 | −1.7 | −2.1 | |
| PC | pyruvate carboxylase | −0.2 | 0.2 | 0.1 | 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abulizi, N.; Quin, C.; Brown, K.; Chan, Y.K.; Gill, S.K.; Gibson, D.L. Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids. Nutrients 2019, 11, 418. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11020418
Abulizi N, Quin C, Brown K, Chan YK, Gill SK, Gibson DL. Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids. Nutrients. 2019; 11(2):418. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11020418
Chicago/Turabian StyleAbulizi, Nijiati, Candice Quin, Kirsty Brown, Yee Kwan Chan, Sandeep K. Gill, and Deanna L. Gibson. 2019. "Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids" Nutrients 11, no. 2: 418. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11020418





