Identification of Education Models to Improve Health Outcomes in Arab Women with Pre-Diabetes
Abstract
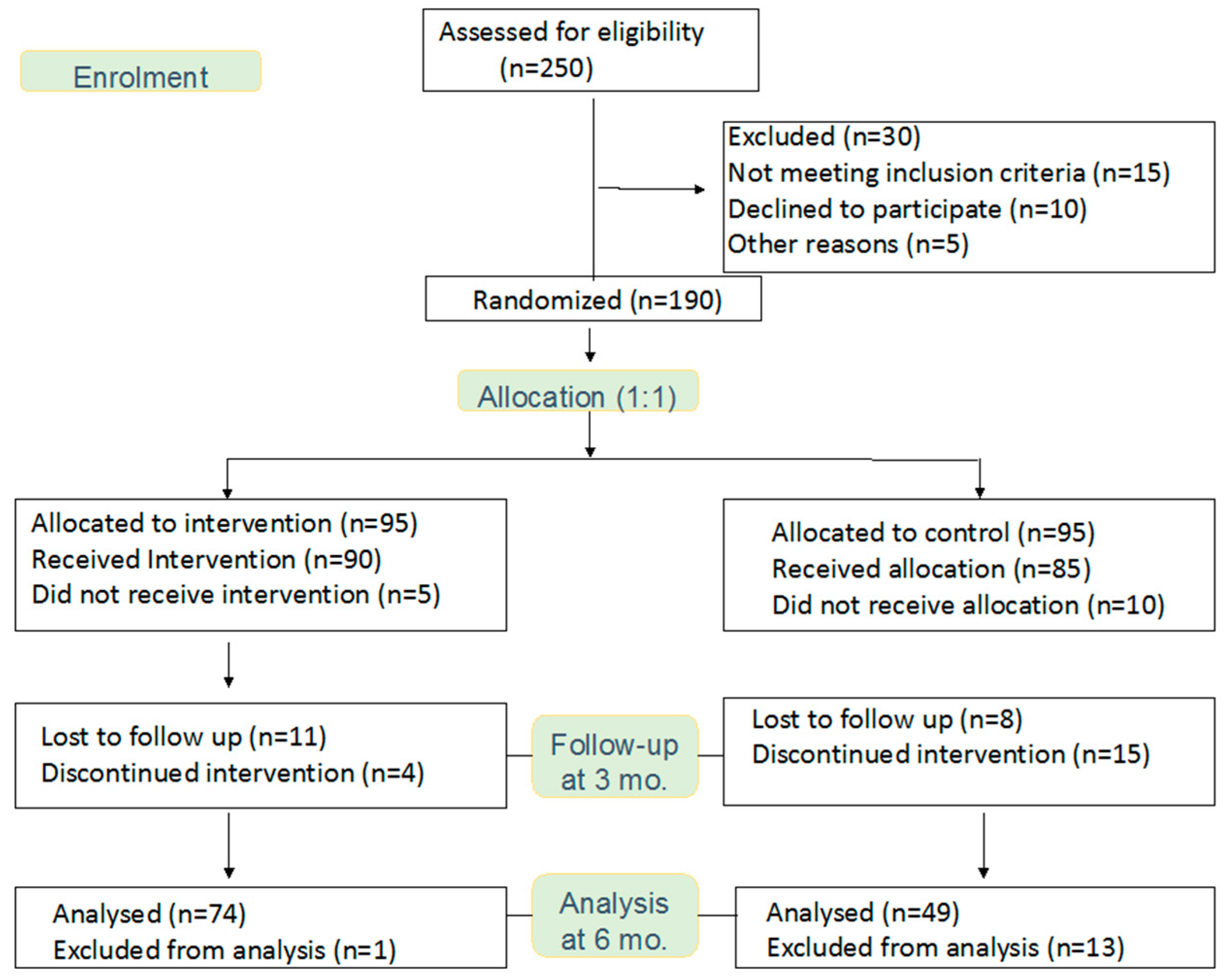
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling Method
2.2. Randomisation
2.3. Data Collection
2.4. Intervention
2.5. Data Analysis
3. Results
3.1. Study Participants
3.2. General Characteristics
3.3. Baseline Clinical and Dietary Intake Differences between Groups
3.4. Clinical Differences between Groups Over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diabetes Mellitus Fact Sheet. Available online: https://www.who.int/mediacentre/factsheets/fs138/en/ (accessed on 26 April 2019).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [CrossRef]
- Yip, W.C.Y.; Sequieira, I.R.; Plank, L.D.; Poppitt, S.D. Prevalence of pre-diabetes across ethnicities: A review of impaired fasting glucose (IFG) and impaired fasting glucose tolerance (IGT) for classification of dysglycaemia. Nutrients 2017, 9, 1273. [Google Scholar] [CrossRef]
- Alotaibi, A.; Perry, L.; Gholizadeh, L.; Al-Ganmi, A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J. Epidemiol. Glob. Health 2017, 7, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zowgar, A.M.; Siddiqui, M.I.; Alattas, K.M. Level of diabetes knowledge among adult patients with diabetes using diabetes knowledge test. Saudi Med. J. 2018, 39, 161–168. [Google Scholar] [CrossRef]
- Aljoudi, A.S.; Taha, A.Z. Knowledge of diabetes risk factors and preventive measures among attendees of a primary care center in eastern Saudi Arabia. Ann. Saudi Med. 2009, 29, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, A.M.; Abo El-Fetoh, N.M.; Alotaibi, H.K.; Alanazi, K.A.; Alotaibi, B.K.; Alshammari, S.M.; Alanazi, S.R.; Alhazmi, M.D.; Alshammari, Y.T.; Alshammari, Z.Q. Survey of awareness of diabetes mellitus among the Arar population, northern border region of Saudi Arabia. Electron. Physician 2017, 9, 5369–5374. [Google Scholar] [CrossRef]
- Al Rasheed, R.; Al Adel, F. Diabetic retinopathy: Knowledge, awareness and practices of physicians in primary-care centers in Riyadh, Saudi Arabia. Saudi J. Ophthalmol. 2017, 31, 2–6. [Google Scholar] [CrossRef]
- Gosadi, I.M.; Goyder, E.C.; Teare, M.D. Investigating the potential effect of consanguinity on type 2 diabetes susceptibility in a Saudi population. Hum. Hered. 2014, 77, 197–206. [Google Scholar] [CrossRef]
- Alfadhil, E.M.; Osman, E.N.; Basri, T.H.; Mansuri, N.S.; Youssef, M.H.; Assaaedi, S.A.; Aljohani, B.A. Gestational diabetes among Saudi women: Prevalence, risk factors and pregnancy outcomes. Ann. Saudi Med. 2015, 35, 222–230. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Tuffaha, M.; Hanlon, M.; El Bcheraoul, C.; Daoud, F.; Al Saeedi, M.; Alrasheedy, A.A.; Al Hussein, M.; Al Memish, Z.A.; Basulaiman, M.; et al. Cost of diabetes in the kingdom of Saudi Arabia 2014. J. Diabetes Metab. 2015, 6, 8. [Google Scholar]
- Diabetes Prevention program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindstrom, J.; Ericksson, J. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R.; Byham-Gray, L.; Denmark, R.; Winkle, P.J. The effect of medical nutrition therapy by a registered dietitian nutritionist in patient with prediabetes participating in a randomized controlled clinical research trial. J. Acad. Nutr. Diet. 2014, 114, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Al-Bannay, H.R.; Jongbloed, L.E.; Jarus, T.; Alabdulwahab, S.S.; Khoja, T.A.; Dean, E. Outcomes of a type 2 diabetes education program adapted to the cultural contexts of Saudi women. A pilot study. Saudi Med. J. 2015, 36, 869–873. [Google Scholar] [CrossRef]
- Mokabel, F.M.; Aboulazm, S.F.; Hassan, H.E.; Al-Qahtani, M.F.; Arashedi, S.F.; Zainuddin, F.A. The efficacy of a diabetic educational program and predictors of compliance of patients with non-insulin-dependent (type 2) diabetes mellitus in Al-Khobar, Saudi Arabia. J. Family Community Med. 2017, 24, 164–172. [Google Scholar] [CrossRef]
- Al-Nozha, M.M.; Al-Maatouq, M.A.; Al-Mazrou, Y.Y.; Al-Harthi, S.S.; Arafah, M.R.; Khalil, M.Z.; Khan, N.B.; Al-Khadra, A.; Al-MArzouki, K.; Nouh, M.S.; et al. Diabetes mellitus in Saudi Arabia. Saudi Med. J. 2004, 25, 1603–1610. [Google Scholar]
- Khattab, A.D.; Aboifotouh, M.A.; Khan, M.Y.; Humaidi, M.A.; Al-Kaldi, Y.M. Compliance and control of diabetes in a family practice setting, Saudi Arabia. East. Mediterr. Health J. 1999, 5, 755–765. [Google Scholar]
- Kamuhabwa, A.R.; Charles, E. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar es Salaam. Drug Health Patient. Saf. 2014, 6, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba-Essa, E.M.; Mobarak, E.I.; Alghamdi, A.; Al-Daghri, N.M. Intensified glucose self-monitoring with education in Saudi DM patients. Int. J. Clin. Exp. Med. 2015, 8, 19374–19380. [Google Scholar]
- Tourkmani, A.M.; Hassali, M.A.; Alharbi, T.J.; Alkhashan, H.I.; Alobikan, A.H.; Bakhiet, A.H.; Alqahtani, H.B.; Alrasheedy, A.A.; Alawwad, A.A.; Alawwad, A.D.; et al. Impact of Ramadan focused educational program on hypoglycemic risk and metabolic control for patients with type 2 diabetes. Patient Prefer. Adherence 2016, 10, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahbi, A.M. Impact of a diabetic foot care education program on lower limb amputation rate. Vasc. Health Risk Manag. 2010, 6, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Al-Musally, R.M.; Al-Sardi, M.A.; El-Elq, Z.A.; Elahi, A.H.; Alduhailan, R.K.; El-Elq, M.A.; Zainuddin, F.A.; Alsafar, N.A.; Altammar, J.A.; Al-Elq, A.H. Health education to diabetic patients before the start of Ramadan: Experience from a teaching hospital in Dammam. J. Family Community Med. 2017, 24, 111–117. [Google Scholar]
- Weisman, A.; Fazil, G.S.; Johns, A.; Booth, G.L. Evolving trends in the epidemiology, risk factors, and prevention of type 2 diabetes: A review. Can. J. Cardiol 2018, 34, 552–564. [Google Scholar] [CrossRef]
- Al-Hazzaa, H.M. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int. J. Health Sci. (Qassim) 2018, 12, 50–64. [Google Scholar]
- Kahan, D. Adult physical inactivity prevalence in the Muslim world: Analysis of 38 countries. Prev. Med. Rep. 2015, 2, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Gossadi, I.M.; Alatar, A.A.; Otayf, M.M.; AlJahani, D.M.; Ghabbani, H.M.; AlRabjan, W.A.; Alrsheed, A.M.; Al-Nasser, K.A. Development of a Saudi food frequency questionnaire and testing its reliability and validity. Saudi Med. J. 2017, 38, 636–641. [Google Scholar] [CrossRef]
- Alfawaz, H.A.; Wani, K.; Alnaami, A.M.; Al-Saleh, Y.; Aljohani, N.J.; Al-Attas, O.S.; Alokail, M.S.; Kumar, S.; Al-Daghri, N.M. Effects of different and lifestyle modification therapies on metabolic syndrome in prediabetic Arab patients: A 12-month longitudinal study. Nutrients 2018, 10, 383. [Google Scholar] [CrossRef]
- Pillay, J.; Armstrong, M.J.; Butalia, S.; Donovan, L.E.; Sigal, R.J.; Vandermeer, B.; Chordiya, P.; Dhakal, S.; Harting, L.; Nuspl, M.; et al. Behavioral programs for type 2 diabetes mellitus: A systematic review and network meta-analysis. Ann. Intern. Med. 2015, 163, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Velasquez-Lopez, L.; Gonzalez-Figueroa, E.; Medina-Bravo, P.; Pineda-Del Aguila, I.; Avila-Jimenez, L.; Ramos-Hernandez, R.; Klunder-Klunder, M.; Escobedo-de la Pena, J. Low calorie and carbohydrate diet: To improve the cardiovascular risk indicators in overweight or obese adults with prediabetes. Endocrine 2013, 43, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Konig, D.; Kookhan, S.; Schaffner, D.; Deibert, P.; Berg, A. A meal replacement regimen improves blood glucose levels in prediabetic healthy individuals with impaired fasting glucose. Nutrition 2014, 30, 1306–1309. [Google Scholar] [CrossRef]
- Briggs Early, K.; Stanley, K. Position of the Academy of Nutrition and Dietetics: The role of medical nutrition therapy and registered dietititian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J. Acad. Nutr. Diet. 2018, 118, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.A.; Choudhari, P.K.; Mahajan, R.R.; Sayyad, M.G.; Pratyush, D.D.; Hasan, I.; Javherani, R.S.; Bothale, M.M.; Purandare, V.B.; Unnikrishnan, A.G. Effects of a low-calorie diet on restoration of normoglycemia in obese subjects with type 2 diabetes. Indian J. Endocrinol. Metab. 2017, 21, 776–780. [Google Scholar] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Chaudhri, O.; Karimi, E.; Nematy, M. The effect of Ramadan fasting on risk factors and anthropometrics parameters: A systematic review. Pak. J. Med. Sci. 2015, 31, 1250–1255. [Google Scholar] [CrossRef]
- Coppell, K.J.; Abel, S.L.; Freer, T.; Gray, A.; Sharp, K.; Norton, J.K.; Spedding, T.; Ward, L.; Whitehead, L.C. The effectiveness of a primary care nursing-led dietary intervention for prediabetes: A mixed methods pilot study. BMC Fam. Pract. 2017, 18, 106. [Google Scholar] [CrossRef]
- Cradock, K.A.; OLaighin, G.; Finucane, F.M.; McKay, R.; Quinlan, L.R.; Martin Ginis, K.A.; Gainforth, H.L. Diet behavior change techniques in type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2017, 40, 1800–1810. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [Green Version]
- Senitan, M.; Alhaiti, A.H.; Gillespie, J.; Alotaibi, B.F.; Lenon, G.B. The referral system between primary and secondary health care in Saudi Arabia for patients with type 2 diabetes: A systematic review. J. Diabetes Res. 2017, 2017, 4183604. [Google Scholar] [CrossRef] [PubMed]
- Ferwana, M.S.; Alshamlan, A.; Al Madani, W.; Al Khateeb, B.; Bawazir, A. Five-year comparison of diabetic control between community diabetic center and primary care centers. J. Family Med. Prim. Care 2016, 5, 641–645. [Google Scholar] [CrossRef] [PubMed]
| Lifestyle Intervention | IG | CG |
|---|---|---|
Baseline
| Explained by registered dietitian individually | Given as a booklet to the group |
| Bimonthly lifestyle education sessions for 3 months | ✔ | None |
| Dietary counselling | Every two weeks for 3 months | None |
| Dietary intake record | Baseline and after 3 months | |
| Physical activity record | ||
| Mode of follow-up | Individualised | Group |
| On demand support system | ✔ | None |
| Blood extraction | Baseline and every 3 months | |
| Anthropometrics | ||
| Parameter | IG | CG | p-Value |
|---|---|---|---|
| Anthropometrics | |||
| BMI (kg/m2) | 31.2 ± 7.0 | 32.3 ± 5.4 | 0.38 |
| Waist (cm) | 88.6 ± 13.5 | 91.3 ± 14.8 | 0.29 |
| Hips (cm) | 105.2 ± 13.4 | 105.4 ± 14.6 | 0.96 |
| Waist-Hip Ratio | 0.84 ± 0.08 | 0.87 ± 0.08 | 0.09 |
| Systolic BP (mmHg) | 122.6 ± 11.4 | 127.9 ± 16.7 | 0.06 |
| Diastolic BP (mmHg) | 77.8 ± 10.8 | 75.6 ± 11.0 | 0.29 |
| Glycaemic Profile | |||
| Glucose (mmol/L) | 5.6 ± 0.9 | 5.4 ± 2.3 | 0.54 |
| HbA1c (%) | 6.0± 0.3 | 6.1 ± 0.29 | 0.052 |
| Lipid Profile | |||
| Triglycerides (mmol/L) | 1.4 ± 0.7 | 1.6 ± 1.0 | 0.21 |
| Total Chol (mmol/L) | 5.1 ± 1.0 | 4.8 ± 0.8 | 0.04 |
| HDL-Chol (mmol/L) | 1.3 ± 0.3 | 1.0 ± 0.4 | <0.001 |
| LDL-Chol (mmol/L) | 3.2 ± 0.9 | 3.0 ± 0.8 | 0.18 |
| Energy Intake | |||
| Energy (kilocalories) | 2490.1 ± 522.9 | 2593.5 ± 519.8 | 0.28 |
| Macronutrients | |||
| Carbohydrate (g) | 389.9 ± 94.8 | 415.2 ± 80.0 | 0.12 |
| Carbohydrate Energy (%) | 62.8 ± 10.3 | 64.5 ± 7.5 | 0.31 |
| Protein (g) | 95.9 ± 58.2 | 109.4 ± 44.8 | 0.17 |
| Protein Energy (%) | 15.3 ± 6.7 | 16.8 ± 5.5 | 0.18 |
| Fat (g) | 60.8 ± 26.8 | 55.0 ± 26.0 | 0.23 |
| Fat Energy (%) | 21.9 ± 8.5 | 18.6 ± 6.2 | 0.02 |
| Parameter | Intervention | Control | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | Baseline | 3 Months | 6 Months | ||
| Anthropometrics | |||||||
| BMI (kg/m2) | 31.2 ± 7.0 | 31.1 ± 7.0 | 30.4 ± 7.1 ab | 32.3 ± 5.4 | 32.0 ± 5.3 | 31.5 ± 5.0 | 0.38 |
| % Weight change | - | −0.14 (6.3) | −2.28 (10.7) b | - | −0.62 (5.2) | −1.82 (10.0) | 0.99 |
| Waist (cm) | 88.6 ± 13.5 | 87.5 ± 14.0 | 85.9 ± 14.0 ab | 91.3 ± 14.8 | 92.6 ± 13.8 | 91.4 ± 15.0 | 0.09 |
| Hips (cm) | 105.2 ± 13.4 | 104.3 ± 13.0 | 103.3 ± 13.6 a | 105.4 ± 14.6 | 106.5 ± 13.0 | 105.9 ± 14.4 | 0.51 |
| Waist-hip ratio | 0.84 ± 0.08 | 0.84 ± 0.08 | 0.83 ± 0.09 | 0.87 ± 0.08 | 0.87 ± 0.06 | 0.86 ± 0.08 | 0.04 |
| Systolic BP (mmHg) | 122.6 ± 11.4 | 122.5 ± 8.7 | 121.9 ± 9.3 | 127.9 ± 16.7 | 127.6 ± 17.4 | 127.4 ± 13.6 | 0.01 |
| Diastolic BP (mmHg) | 77.8 ± 10.8 | 77.6 ± 7.5 | 78.3 ± 10.7 | 75.6 ± 11.0 | 77.8 ± 11.1 | 80.1 ± 8.3 | 0.96 |
| Glycaemic Profile | |||||||
| Glucose (mmol/L) | 5.6 ± 0.9 | 5.6 ± 0.8 | 5.2 ± 0.8 ab | 5.4 ± 2.3 | 5.3 ± 1.3 | 5.4 ± 1.1 | 0.79 |
| Hba1c (%) | 6.0 ± 0.3 | 6.0 ± 0.3 | 5.8 ± 0.3 ab | 6.1 ± 0.29 | 6.1 ± 0.4 | 6.3 ± 0.4 ab | <0.001 |
| Lipid Profile | |||||||
| Triglycerides (mmol/L) | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.4 ±0.6 | 1.6 ± 1.0 | 1.5 ± 0.6 | 1.4 ± 0.5 a | 0.31 |
| Total Chol (mmol/L) | 5.1 ± 1.0 | 5.0 ± 1.0 | 4.7 ± 1.0 ab | 4.8 ± 0.8 | 4.6 ± 0.8 | 4.5 ± 0.8 a | 0.04 |
| HDL-Chol (mmol/L) | 1.3 ± 0.3 | 1.4 ± 0.4 a | 1.8 ± 0.5 ab | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.1 ± 0.3 | <0.001 |
| LDL-Chol (mmol/L) | 3.2 ± 0.9 | 3.0 ± 0.9 a | 2.3 ± 1.1 ab | 3.0 ± 0.8 | 2.9 ± 0.7 | 2.8 ± 0.8 | 0.73 |
| Energy Intake | |||||||
| Energy kilocalories | 2490 ± 523 | 2226 ± 488 a | 2055 ± 462 ab | 2594 ± 520 | 2724 ± 581 a | 2708 ± 536 a | <0.001 |
| Macronutrients | |||||||
| Carbohydrate (g) | 389.9 ± 94.8 | 347.9 ± 93.4 a | 322.2 ± 93.7 ab | 415.2 ± 80.0 | 415.8 ± 85.4 | 416.3 ± 81.6 | <0.001 |
| CHO energy (%) | 62.8 ± 10.3 | 62.4 ± 10.2 | 62.3 ± 9.9 | 64.5 ± 7.5 | 61.7 ± 8.5 a | 62.2 ± 10.2 a | 0.86 |
| Protein (g) | 95.9 ± 58.2 | 87.5 ± 52.0 a | 80.2 ± 42.3 ab | 109.4 ± 44.8 | 143.2 ± 69.4 a | 143.9 ± 69.0 a | <0.001 |
| Protein energy (%) | 15.3 ± 6.7 | 15.7 ± 7.1 a | 15.8 ± 6.5 | 16.8 ± 5.5 | 20.7 ± 7.5 a | 21.0 ± 8.9 a | 0.001 |
| Fat (g) | 60.8 ± 26.8 | 53.9 ± 22.6 a | 49.5 ± 19.8 ab | 55.0 ± 26.0 | 54.2 ± 26.0 | 51.9 ± 25.8 a | 0.82 |
| Fat energy (%) | 21.9 ± 8.5 | 21.8 ± 7.8 | 21.9 ± 8.0 | 18.6 ± 6.2 | 17.6 ± 6.3 a | 16.7 ± 6.1 ab | 0.002 |
| Life style | |||||||
| Physical act (freq) | 3.2 ± 1.4 | 3.3 ± 1.3 | 3.2 ± 1.3 | 3.1 ± 1.4 | 2.9 ± 1.3 | 3.1 ± 1.4 | 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamdan, R.; Avery, A.; Salter, A.; Al-Disi, D.; Al-Daghri, N.M.; McCullough, F. Identification of Education Models to Improve Health Outcomes in Arab Women with Pre-Diabetes. Nutrients 2019, 11, 1113. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051113
Al-Hamdan R, Avery A, Salter A, Al-Disi D, Al-Daghri NM, McCullough F. Identification of Education Models to Improve Health Outcomes in Arab Women with Pre-Diabetes. Nutrients. 2019; 11(5):1113. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051113
Chicago/Turabian StyleAl-Hamdan, Rasha, Amanda Avery, Andrew Salter, Dara Al-Disi, Nasser M. Al-Daghri, and Fiona McCullough. 2019. "Identification of Education Models to Improve Health Outcomes in Arab Women with Pre-Diabetes" Nutrients 11, no. 5: 1113. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051113






