Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
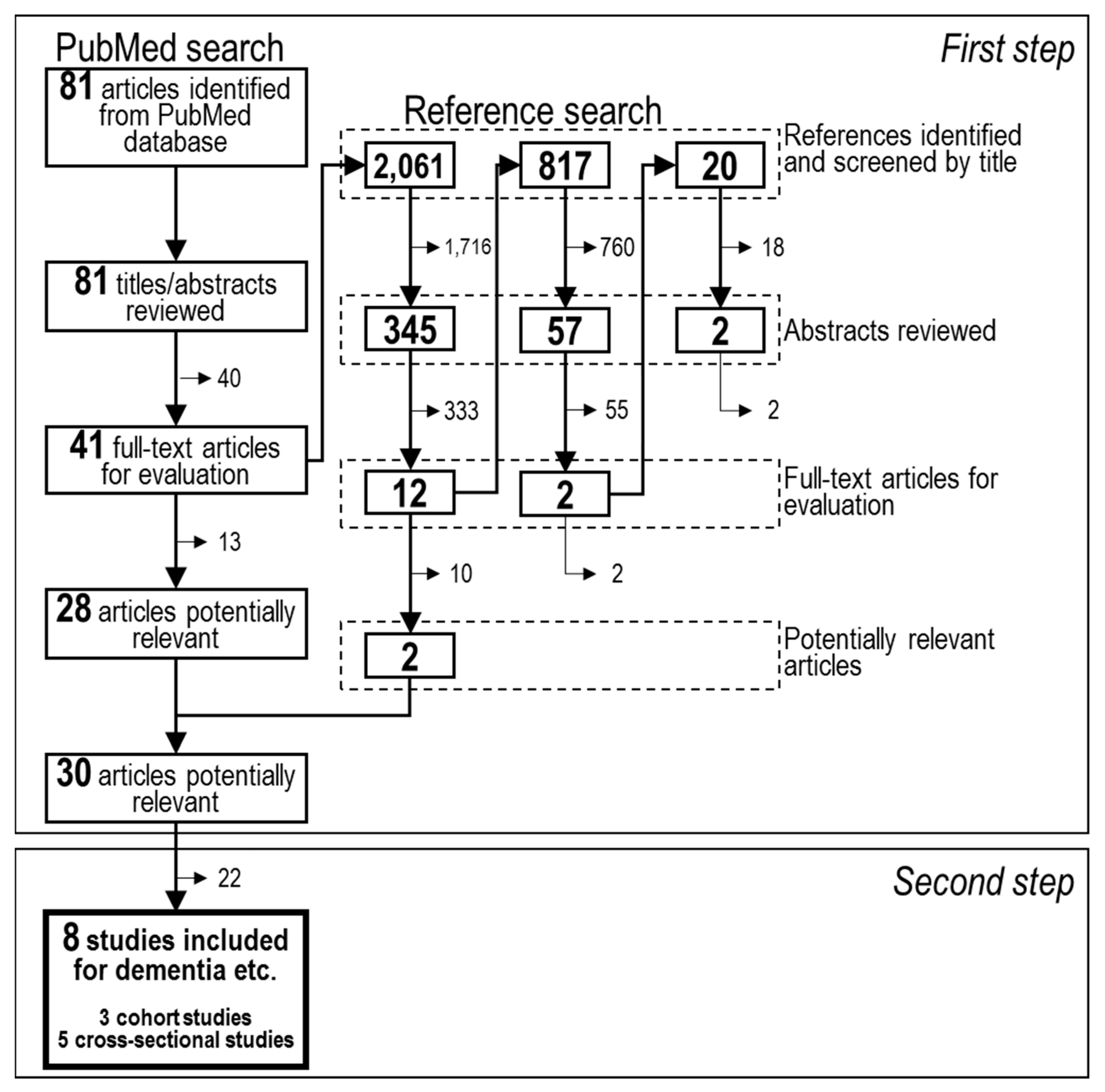
2.1. Search Strategy
2.2. Study Selection
2.3. Study Quality Assessment
2.4. Data Extraction
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Dementia, Fact Sheet on Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 15 February 2019).
- Santos, C.; Costa, J.; Santos, J.; Vaz-Carneiro, A.; Lunet, N. Caffeine intake and dementia: Systematic review and meta-analysis. J. Alzheimers Dis. 2010, 20, S187–S204. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kwak, S.M.; Myung, S.-K. Caffeine intake from coffee or tea and cognitive disorders: A meta-analysis of observational studies. Neuroepidemiology 2015, 44, 51–63. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a Potential Therapeutic Candidate for the Treatment and Management of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health’s National Library of Medicine. PubMed. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed/ (accessed on 15 February 2019).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Green Tea Consumption and the Risk of Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Geriatr. Psychiatry 2016, 24, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Noguchi-Shinohara, M.; Yuki, S.; Dohmoto, C.; Ikeda, Y.; Samuraki, M.; Iwasa, K.; Yokogawa, M.; Asai, K.; Komai, K.; Nakamura, H.; et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS ONE 2014, 9, e96013. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Yuan, Y.; Zhang, X.; Zuo, X.; Cui, L.; Liu, Y.; Chen, W.; Su, N.; Wang, H.; et al. Gender differences in the protective effects of green tea against amnestic mild cognitive impairment in the elderly Han population. Neuropsychiatr. Dis. Treat. 2018, 14, 1795–1801. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Sun, Y.; Lee, H.-J.; Chen, T.-F.; Wang, P.-N.; Lin, K.-N.; Tang, L.-Y.; Lin, C.-C.; Chiu, M.-J. Modest Overweight and Healthy Dietary Habits Reduce Risk of Dementia: A Nationwide Survey in Taiwan. J. Prev. Alzheimers Dis. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Kitamura, K.; Watanabe, Y.; Nakamura, K.; Sanpei, K.; Wakasugi, M.; Yokoseki, A.; Onodera, O.; Ikeuchi, T.; Kuwano, R.; Momotsu, T.; et al. Modifiable Factors Associated with Cognitive Impairment in 1,143 Japanese Outpatients: The Project in Sado for Total Health (PROST). Dement Geriatr. Cognit. Disord. Extra 2016, 6, 341–349. [Google Scholar] [CrossRef]
- Shen, W.; Xiao, Y.; Ying, X.; Li, S.; Zhai, Y.; Shang, X.; Li, F.; Wang, X.; He, F.; Lin, J. Tea Consumption and Cognitive Impairment: A Cross-Sectional Study among Chinese Elderly. PLoS ONE 2015, 10, e0137781. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef]
- Luca, M.; Luca, A.; Calandra, C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxid. Med. Cell. Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Kumamoto, M.; Sonda, T.; Nagayama, K.; Tabata, M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci. Biotechnol. Biochem. 2001, 65, 126–132. [Google Scholar] [CrossRef]
- Henning, S.M.; Niu, Y.; Lee, N.H.; Thames, G.D.; Minutti, R.R.; Wang, H.; Go, V.L.; Heber, D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004, 80, 1558–1564. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.L.; Zhang, X.Y.; Lv, C.; Li, J.; Yuan, Y.; Yin, F.X. Pharmacokinetics and blood-brain barrier penetration of (+)-catechin and (−)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J. Agric. Food Chem. 2012, 60, 9377–9383. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; O’Brien, J.; Weuve, J.; Blacker, D.; Metti, A.L.; Yaffe, K. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.L.; Wolters, F.J.; Ikram, M.A.; de Wolf, F.; Bos, D.; Hofman, A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement. 2018, 14, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L.; Stine, W.B., Jr.; Teplow, D.B. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol. Aging 2004, 25, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Lorenz, M.; Stangl, K. The role of tea and tea flavonoids in cardiovascular health. Mol. Nutr. Food Res. 2006, 50, 218–228. [Google Scholar] [CrossRef]
- Fraser, M.L.; Mok, G.S.; Lee, A.H. Green tea and stroke prevention: Emerging evidence. Complement. Ther. Med. 2007, 15, 46–53. [Google Scholar] [CrossRef]
- Moore, R.J.; Jackson, K.G.; Minihane, A.M. Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 2009, 102, 1790–1802. [Google Scholar] [CrossRef]
- Bielli, A.; Scioli, M.G.; Mazzaglia, D.; Doldo, E.; Orlandi, A. Antioxidants and vascular health. Life Sci. 2015, 143, 209–216. [Google Scholar] [CrossRef]
- Song, J.; Xu, H.; Liu, F.; Feng, L. Tea and cognitive health in late life: Current evidence and future directions. J. Nutr. Health Aging 2012, 16, 31–34. [Google Scholar] [CrossRef]
- Polito, C.A.; Cai, Z.-Y.; Shi, Y.-L.; Li, X.-M.; Yang, R.; Shi, M.; Li, Q.-S.; Ma, S.-C.; Xiang, L.-P.; Wang, K.-R.; et al. Association of Tea Consumption with Risk of Alzheimer’s Disease and Anti-Beta-Amyloid Effects of Tea. Nutrients 2018, 10, 655. [Google Scholar] [CrossRef]
| Number | Items | Terms |
|---|---|---|
| #1 | Humans | Humans[mesh] OR people[tiab] OR participants[tiab] OR men[tiab] OR women[tiab] OR population[tiab] OR populations[tiab] OR individuals[tiab] OR people[ot] OR participants[ot] OR men[ot] OR women[ot] OR population[ot] OR populations[ot] OR individuals[ot] |
| #2 | Study designs | Epidemiologic Studies[mesh] OR “case control”[tiab] OR cohort[tiab] OR cohorts[tiab] OR “cross sectional”[tiab] OR “longitudinal study”[tiab] OR “longitudinal studies”[tiab] OR “longitudinal trial”[tiab] OR “longitudinal trials”[tiab] OR “prospective study”[tiab] OR “prospective studies”[tiab] OR “prospective trial”[tiab] OR “prospective trials”[tiab] OR “retrospective study”[tiab] OR “retrospective studies”[tiab] OR “retrospective trial”[tiab] OR “retrospective trials”[tiab] OR “case control”[ot] OR cohort[ot] OR cohorts[ot] OR “cross sectional”[ot] OR “longitudinal study”[ot] OR “longitudinal studies”[ot] OR “longitudinal trial”[ot] OR “longitudinal trials”[ot] OR “prospective study”[ot] OR “prospective studies”[ot] OR “prospective trial”[ot] OR “prospective trials”[ot] OR “retrospective study”[ot] OR “retrospective studies”[ot] OR “retrospective trial”[ot] OR “retrospective trials”[ot] |
| #3 | Exposure | tea[mesh] OR tea[tiab] OR teas[tiab] OR tea[ot] OR teas[ot] |
| #4 | Relevant outcomes | Dementia[mesh] OR Cognition Disorders[mesh] OR dementia[tiab] OR “cognition disorder”[tiab] OR “cognition disorders”[tiab] OR “cognitive disorder”[tiab] OR “cognitive disorders”[tiab] OR “cognition impairment”[tiab] OR “cognition impairment”[tiab] OR “cognitive impairment”[tiab] OR “cognitive impairments”[tiab] OR “cognition decline”[tiab] OR “cognitive decline”[tiab] OR “cognition dysfunction”[tiab] OR “cognitive dysfunction”[tiab] OR “cognition function”[tiab] OR “cognition functions”[tiab] OR “cognitive function”[tiab] OR “cognitive functions”[tiab] OR dementia[ot] OR “cognition disorder”[ot] OR “cognition disorders”[ot] OR “cognitive disorder”[ot] OR “cognitive disorders”[ot] OR “cognition impairment”[ot] OR “cognition impairment”[ot] OR “cognitive impairment”[ot] OR “cognitive impairments”[ot] OR “cognition decline”[ot] OR “cognitive decline”[ot] OR “cognition dysfunction”[ot] OR “cognitive dysfunction”[ot] OR “cognition function”[ot] OR “cognition functions”[ot] OR “cognitive function”[ot] OR “cognitive functions”[ot] |
| Term Combination |
|---|
| #1 AND #2 AND #3 AND #4 |
| First Author, Publication Year, [Reference No.] | Study | Subjects | Exposure Assessment | Outcome Assessment | Adjustment for Potential Confounders | Main Findings | Quality Assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| STROBE Score | Study Quality | |||||||||
| Fischer K, 2018 [10] | AgeCoDe and AgeQualiDe, German, 2003-ongoing (≥10 years follow-up). | 2622 of 22,701 primary care patients living in the urban areas of the 6 German cities (Bonn, Düsseldorf, Hamburg, Leipzig, Mannheim, or Munich), aged ≥75 years. | Self-administered questionnaire of frequency at FU-1 using a short and concise 8-item “cognitive health” food intake screener. | AD according to SIDAM with consensus of the interviewing investigator and an experienced geriatrician or geriatric psychiatrist. | Age, gender, BMI, education, APOE ε4 carrier status, smoking status, physical activity score, depression, hypercholesterolemia, and a modified CCI score. | Green tea consumption | AD HR (95% CI) | p | 21 | Medium |
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.94 (0.86; 1.02) | 0.129 | ||||||||
| Tomata Y, 2016 [11] | Ohsaki Cohort 2006 Study, Japan, 2006–2012 (5.7 years follow-up, 67,551 person-years). | 13,645 of 31,694 residents in Ohsaki City, Miyagi Prefecture, northeastern Japan, aged ≥65 years on 1 December 2006. | Self-administered FFQ (Spearman rank correlation coefficient between FFQ and food records was 0.71 for men and 0.53 for women). | Dementia defined as disabling dementia according to the criteria of LTCI system used in Japan. | Age, gender, history of disease (stroke, myocardial infarction, hypertension, diabetes, arthritis, osteoporosis, fracture), education, smoking, alcohol drinking, BMI, psychological distress score, time spent walking, social support, participation in community activities, motor function score, consumption volume of specific foods (green and yellow vegetables and fruit), coffee consumption, and energy intake. | Green tea consumption | Dementia HR (95% CI) | p for trend | 18 | High |
| <1 cup/day | 1 | <0.001 | ||||||||
| 1–2 cups/day | 1.06 (0.89–1.27) | |||||||||
| 3–4 cups/day | 0.88 (0.74–1.04) | |||||||||
| ≥5 cups/day | 0.73 (0.61–0.87) | |||||||||
| Noguchi-Shinohara M, 2014 [12] | Nakajima Project, Japan, 2007–2013 (mean 4.9 (0.9) years follow-up). | 723 of 2845 residents of Nakajima, aged ≥60, completed cognitive tests, without dementia, MCI, or MMSE score <24. | Self-administered questionnaire of frequency, reviewed by trained researchers. | Dementia: DSM-III-R MCI: general MCI criteria of International Working Group. | Age, gender, history of hypertension/diabetes mellitus/hyperlipidemia, education, ApoE, alcohol drinking, smoking, physical activities/hobbies, and coffee/black tea/green tea consumption. | Green tea consumption | Dementia OR (95% CI) | p | 21 | Medium |
| None | 1 | - | ||||||||
| 1–6 d/w | 0.90 (0.34, 2.35) | 0.824 | ||||||||
| Every day | 0.26 (0.06, 1.06) | 0.06 | ||||||||
| Green tea consumption | MCI or dementia OR (95% CI) | p | ||||||||
| None | 1 | - | ||||||||
| 1–6 d/w | 0.47 (0.25, 0.86) | 0.015 | ||||||||
| Every day | 0.32 (0.16, 0.64) | 0.001 | ||||||||
| First Author, Publication Year, [Reference No.] | Study | Subjects | Exposure Assessment | Outcome Assessment | Adjustment for Potential Confounders | Main Findings | Quality Assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| STROBE Score | Study Quality | |||||||||
| Xu H, 2018 [13] | CLAS, China, 2011–2012. | 1003 of randomly selected 4411 residents from 20 target communities in the eastern, mid, and western parts of China, aged ≥60. | Unclear (reviewed type of tea consumed with frequency of tea consumption). | aMCI diagnostic criteria reported by Petersen with MMSE, MoCA, ADL, GDS, HIS, and MRI scans. | Education. | Green tea consumption | MCI OR (95% CI) | p | 11 | Low |
| All male | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.657 (0.46–0.93) | 0.019 | ||||||||
| 55–69 years male | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.376 (0.20–0.70) | 0.002 | ||||||||
| 70–79 years male | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.802 (0.64–1.79) | 0.802 | ||||||||
| ≥80 years male | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.652 (0.28–1.51) | 0.318 | ||||||||
| All female | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.82 (0.58–1.16) | 0.261 | ||||||||
| 55–69 years female | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 1.06 (0.62–1.80) | 0.840 | ||||||||
| 70–79 years female | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.96 (0.56–1.65) | 0.890 | ||||||||
| ≥80 years female | ||||||||||
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.43 (0.18–1.03) | 0.057 | ||||||||
| Lee CY, 2017 [14] | A nationwide, population-based, door-to-door, in-person survey in Taiwan, 2011–2013. | 7964 of 28,600 residents across Taiwan, aged ≥65. | Interview using a structured questionnaire, conducted by well-trained field interviewers according to an operational manual. | All-cause dementia: the core clinical criteria recommended by NIA-AA. | Age, gender, education, BMI, dietary habits, habitual exercises, and co-morbidities, including hypertension, diabetes, and cerebrovascular diseases. | Green tea consumption | All-cause dementia OR (95% CI) | p | 18 | Low |
| Non-consumption | 1 | - | ||||||||
| Consumption | 0.51 (0.34-0.75) | 0.00 | ||||||||
| Kitamura K, 2016 [15] | PROST, Japan, 2008–2014. | 1143 of 2161 patient registry of Sado General Hospital, aged ≥40, not undergoing kidney dialysis. | Self-administered questionnaire of frequency. | Cognitive impairment: MMSE score <24 (MMSE cutoff score of 23/24). | Age, BMI, history of stroke and myocardial infarction, walking time, alcohol, and fruit consumption. | Green tea consumption | Cognitive impairment OR (95% CI) | p | 19 | Low |
| 0 = none, 1 = 1–6 times/wk, 2 = 7 times/wk | 0.83 (0.70–0.98) | 0.032 | ||||||||
| Shen W, 2015 [16] | ZPHS, China, 2014. | 9375 of randomly selected 1500 residents from each of 7 sites in Zhejian province, aged ≥60. | Self-reported frequency/type/volume/preferred concentration in interview by trained researchers. | Cognitive impairment (CCM): MMSE score <18 for illiteracy, <21 for 0–6 years educated, <25 for >6 year educated Cognitive impairment (worldwide): MMSE score <24. | Age, gender, ethnicity, education, marital status, BMI, WHR, SBP, DBP, income, having children, diabetes/CHD/AD/PD, family diabetes/CHD/AD/PD history, smoking, alcohol drinking, activity, vegetable intake, fruit intake, red meat intake, bean intake, milk intake, supplement use, depression, ADL (all analyses), tea types, tea concentration (Tea consumption volume), tea consumption volume, tea concentration (Tea types), tea consumption volume, and tea types (Tea concentration). | Tea types | Cognitive impairment (CCM) OR (95% CI) | p | 19 | Low |
| Non-consumption | 1 | - | ||||||||
| Green tea | 1.04 (0.72, 1.51) | Not shown | ||||||||
| Kuriyama S, 2006 [17] | Tsurugaya Project, Japan, 2002. | 1103 of 2730 residents of Tsurugaya, aged ≥70, with information on tea consumption, cognitive function, body weight, height, blood glucose, blood pressure, depressive symptoms. | Self-administered semi-quantitative questionnaire. | Cognitive impairment: MMSE score <26. | Age, gender, green tea/black or oolong tea consumption, coffee consumption, diabetes mellitus, hypertension, history of stroke, depressive symptoms, education, visiting friends, energy intake, VC/VE supplementation, and fish intake. | Green tea consumption | Cognitive impairment OR (95% CI) | p for trend | 19 | Low |
| ≤3 cups/w | 1 | 0.0006 | ||||||||
| 4–6 cups/w or 1 cup/d | 0.62 (0.33, 1.19) | |||||||||
| ≥2 cups/d | 0.46 (0.30, 0.72) | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakutani, S.; Watanabe, H.; Murayama, N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients 2019, 11, 1165. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051165
Kakutani S, Watanabe H, Murayama N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients. 2019; 11(5):1165. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051165
Chicago/Turabian StyleKakutani, Saki, Hiroshi Watanabe, and Norihito Murayama. 2019. "Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review" Nutrients 11, no. 5: 1165. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051165




