Dietary Flaxseed as a Strategy for Improving Human Health
Abstract
:1. Introduction
2. Flaxseed and Its Use in the Diet
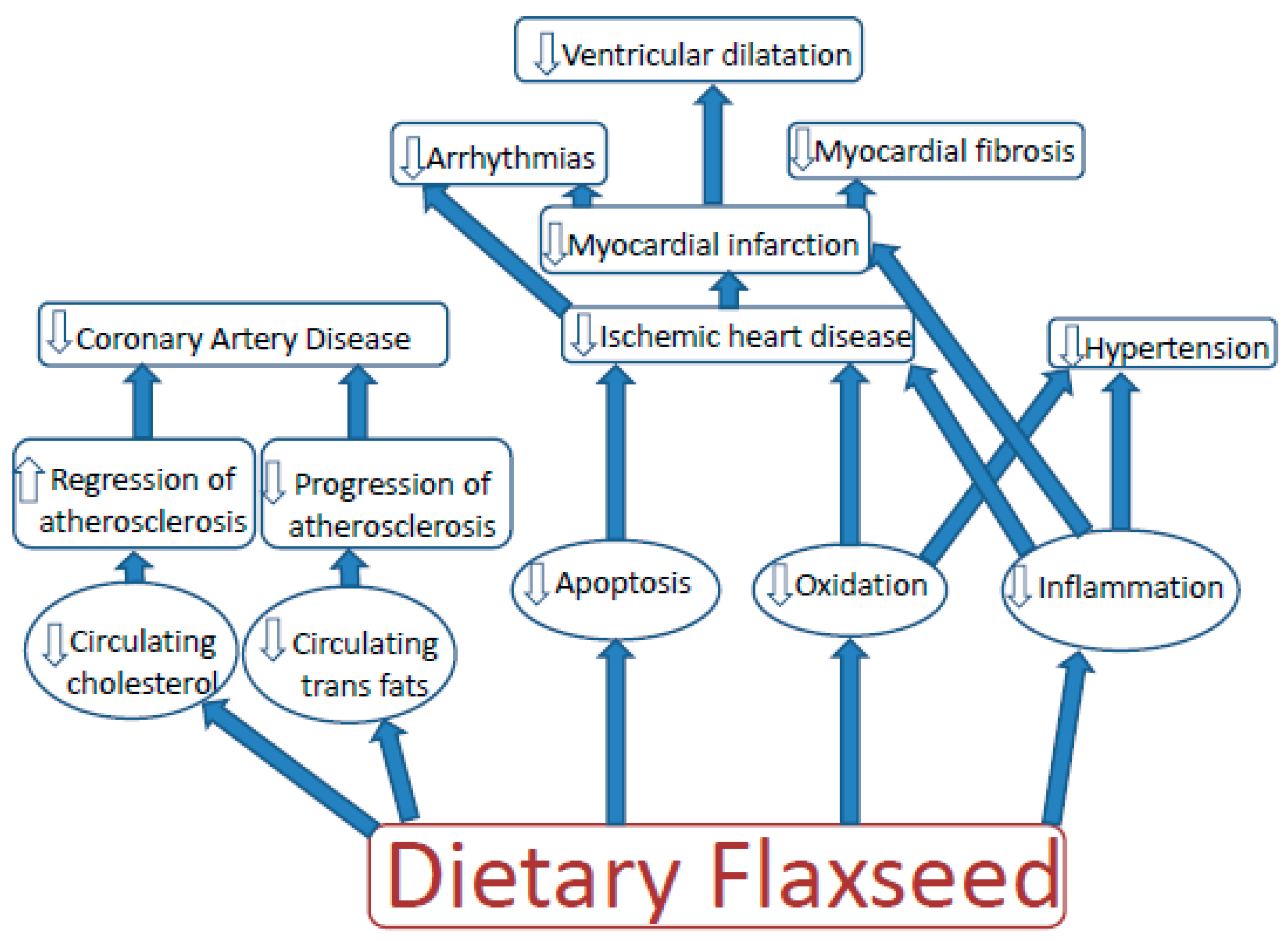
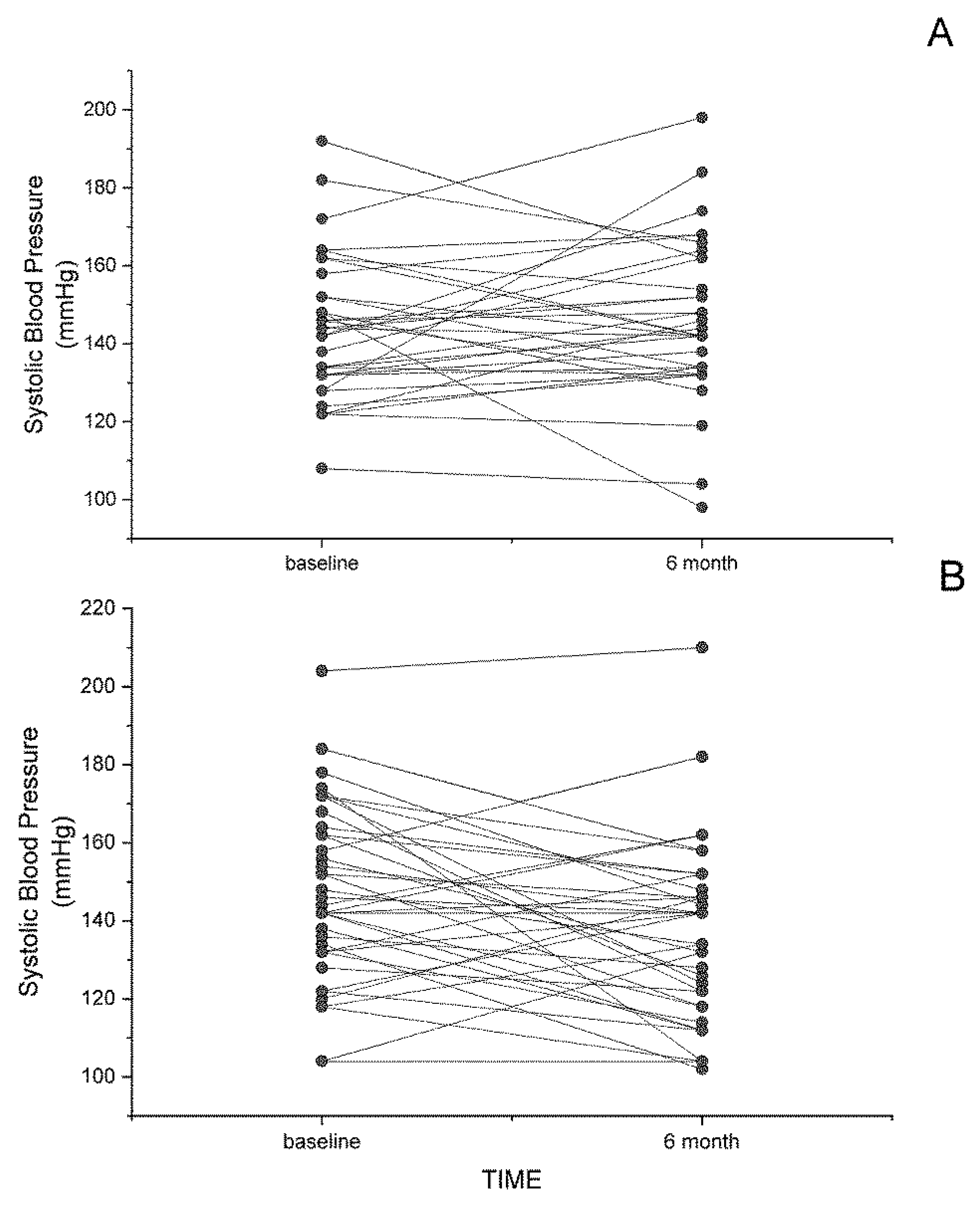
3. Dietary Flaxseed and Cardiovascular Disease
4. Dietary Flaxseed and Diabetes
5. Dietary Flaxseed and Cancers
6. Dietary Flaxseed and the Brain
7. Dietary Flaxseed and Female Hormonal Status
8. Dietary Flaxseed and Skin Health
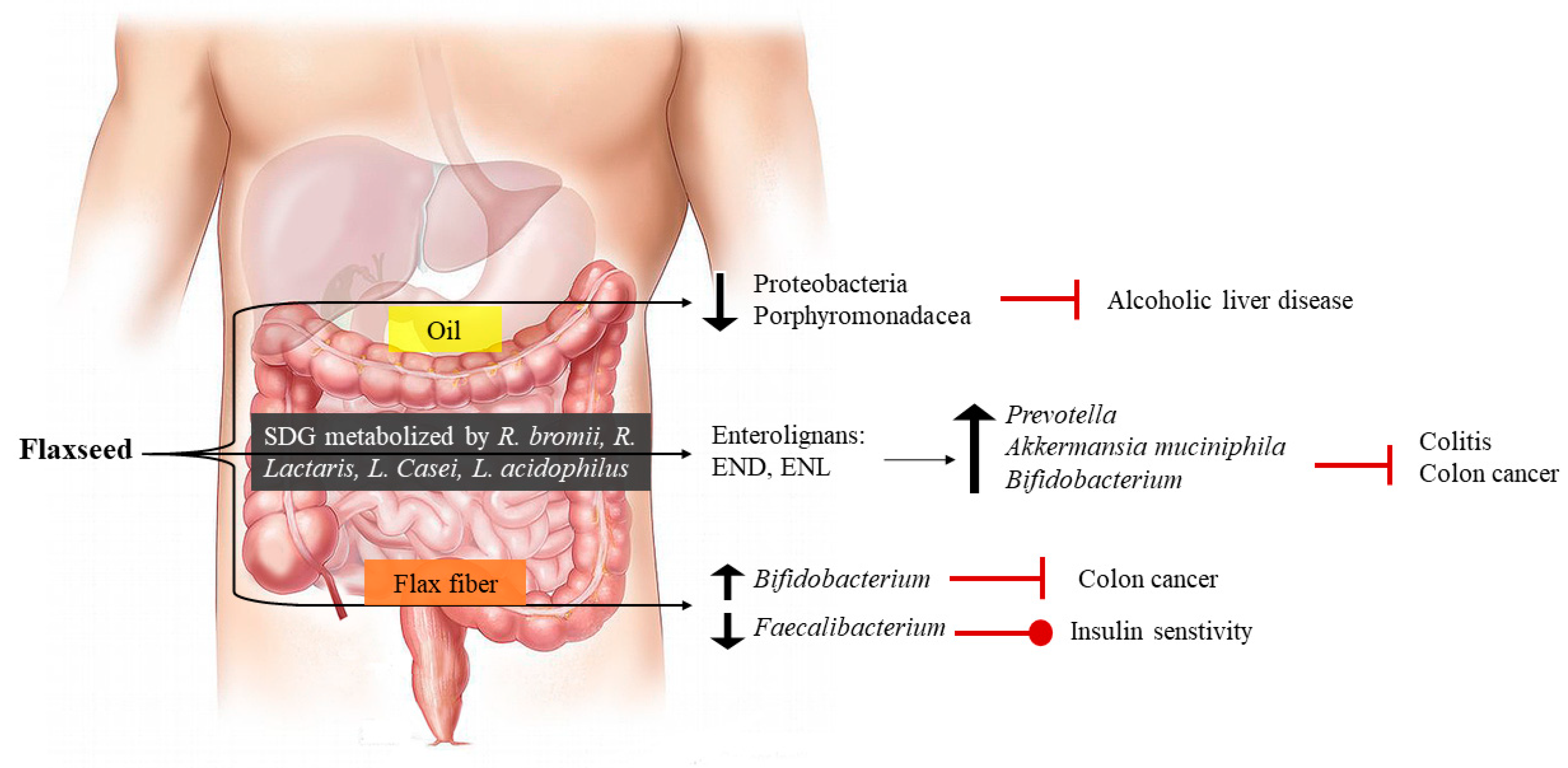
9. Dietary Flaxseed and Gastro-Intestinal Health
10. Toxicity of Flaxseed
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Pierce, G.N. A review of the relative efficacy of dietary, nutritional supplements, lifestyle and drug therapies in the management of hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3508–3527. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef] [PubMed]
- Ramicharitrar, A.; Badrie, N.; Mattfeldt-Beman, M.; Matsuo, H.; Ridley, C. Consumer acceptability of muffins with flaxseed (Linum usitatissimum). J. Food Sci. 2005, 70, 5504–5507. [Google Scholar]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; La Vallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent anti-hypertensive action of dietary flaxseed in hypertensive patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef]
- Pohjanheimo, T.A.; Hakala, M.A.; Tahvonen, R.L.; Salminen, S.J.; Kallio, H.P. Flaxseed in breadmaking: Effects on sensory quality, aging, and composition of bakery products. J. Food Sci. 2006, 71, S343–S348. [Google Scholar] [CrossRef]
- Aliani, M.; Ryland, D.; Pierce, G.N. Effect of flax addition on the flavor profile of muffins and snack bars. Food Res. Int. 2011, 44, 2489–2496. [Google Scholar] [CrossRef]
- Dodin, S.; Lemay, A.; Jacques, H.; Légaré, F.; Forest, J.C.; Mâsse, B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women; a randomized, double-blind, wheat germ placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 1390–1397. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ratnayake, W.M.N.; Cunnane, S.C. Oxidative stability of flaxseed lipids during baking. J. Am. Oil Chem. Soc. 1994, 71, 629–632. [Google Scholar] [CrossRef]
- Aliani, M.; Ryland, D.; Pierce, G.N. Effect of flax addition on the flavor profile and acceptability of bagels. J. Food Sci. 2012, 71, S62–S79. [Google Scholar] [CrossRef]
- Austria, J.A.; Aliani, M.; Malcolmson, L.J.; Dibrov, E.; Blackwood, E.P.; Maddaford, T.G.; Guzman, R.; Pierce, G.N. Daily food choices over one year when patient diets are supplemented with milled flaxseed. J. Funct. Foods 2016, 26, 772–780. [Google Scholar] [CrossRef]
- Edel, A.E.; Aliani, M.; Pierce, G.N. Stability of bioactives in flaxseed and flaxseed-fortified foods. Food Res. Int. 2015, 77, 140–155. [Google Scholar] [CrossRef]
- Austria, J.A.; Richard, M.N.; Chahine, M.N.; Edel, A.L.; Malcolmson, L.J.; Dupasquier, C.M.C.; Pierce, G.N. Bioavailability of alpha linolenic acid in subjects after ingestion of three different forms of flaxseed. J. Am. Coll. Nutr. 2008, 27, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.C.; Weber, A.-M.; Ander, B.P.; Rampersad, P.P.; Steigerwald, S.; Wigle, J.T.; Mitchell, R.W.; Kroeger, E.A.; Gilchrist, J.S.C.; Moghadasian, M.M.; et al. The effects of dietary flaxseed on vascular contractile function and atherosclerosis in rabbits during prolonged hypercholesterolemia. Am. J. Physiol. 2006, 291, H2987–H2996. [Google Scholar]
- Dupasquier, C.M.C.; Dibrov, E.; Kneesh, A.L.; Cheung, P.K.M.; Lee, K.G.Y.; Alexander, H.K.; Yeganeh, B.; Moghadasian, M.H.; Pierce, G.N. Dietary flaxseed inhibits atherosclerosis in the LDL receptor deficient mouse in part through anti-proliferative and anti-inflammatory actions. Am. J. Physiol. 2007, 293, H2394–H2402. [Google Scholar]
- Bassett, C.M.C.; McCullough, R.S.; Edel, A.L.; Patenaude, A.; La Vallee, R.; Pierce, G.N. The alpha linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans-fat. Am. J. Physiol. 2011, 301, H2220–H2226. [Google Scholar]
- Parikh, M.; Raj, P.; Austria, J.A.; Yu, L.; Garg, B.; Netticadan, T.; Pierce, G.N. Dietary flaxseed protects against ventricular arrhythmias and left ventricular dilation after a myocardial infarction. J. Nutr. Biochem. 2019, in press. [Google Scholar]
- Francis, A.A.; Austria, J.A.; La Vallee, R.K.; Deniset, J.F.; Maddaford, G.G.; Dibrov, E.; Pierce, G.N. The effects of dietary flaxseed on atherosclerotic plaque regression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1743–H1751. [Google Scholar] [CrossRef]
- Ander, B.P.; Weber, A.R.; Rampersad, P.; Gilchrist, J.S.C.; Pierce, G.N.; Lukas, A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia- reperfusion in normal and hypercholesterolemic rabbits. J. Nutr. 2004, 134, 3250–3256. [Google Scholar] [CrossRef]
- Ander, B.P.; Hurtado, C.; Raposo, C.S.; Maddaford, T.G.; Deniset, J.F.; Hryshko, L.V.; Pierce, G.N.; Lukas, A. Differential sensitivities of the NCX 1.1 and NCX 1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc. Res. 2007, 73, 395–403. [Google Scholar] [CrossRef]
- Prasad, K. Flax lignan complex slows down the progression of atherosclerosis in hyperlipidemic rabbits. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 38–48. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Rodriguez-Leyva, D.; Aukema, H.; Ravandi, A.; Weighell, W.; Guzman, R.; Pierce, G.N. Dietary flaxseed reduces central aortic blood pressure without cardiac involvement but through changes in plasma oxylipins. Hypertension 2016, 68, 1031–1038. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Zahradka, P.; Ramjiawan, B.; Guzman, R.; Aliani, M.; Pierce, G.N. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral arterial disease: The rationale and design of the FlaxPAD randomized controlled trial. Contemp. Clin. Trials 2011, 32, 724–730. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Aukema, H.M.; Ravandi, A.; Guzman, R.; Dibrov, E.; Pierce, G.N. Flaxseed consumption reduces blood pressure in hypertensive patients by altering circulating oxylipins via an alpha linolenic acid-induced inhibition of soluble epoxide hydrolase. Hypertension 2014, 64, 53–59. [Google Scholar] [CrossRef]
- Ogawa, A.; Suzuki, Y.; Aoyama, T.; Takeuchi, H. Dietary α-linolenic acid inhibits angiotensin-converting enzyme activity and mRNA expression levels in the aorta of spontaneously hypertensive rats. J. Oleo Sci. 2009, 58, 355–360. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Adebiyi, A.P.; Doyen, A.; Li, H.; Bazinet, L.; Aluko, R.E. Low molecular weight flaxseed protein-derived arginine-containing peptides reduced blood pressure of spontaneously hypertensive rats faster than amino acid form of arginine and native flaxseed protein. Food Chem. 2012, 132, 468–475. [Google Scholar] [CrossRef]
- Sawant, S.H.; Bodhankar, S.L. Flax lignan concentrate reverses alterations in blood pressure, left ventricular functions, lipid profile and antioxidant status in DOCA-salt induced renal hypertension in rats. Renal Fail. 2016, 38, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B.; Velasquez, M.T. Potential effects of lignan-enriched flaxseed powder on bodyweight, visceral fat, lipid profile, and blood pressure in rats. Fitoterapia. 2012, 83, 941–946. [Google Scholar] [CrossRef]
- Soltanian, N.; Janghorbani, M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr. Metab. 2018, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Mani, U.V.; Mani, I.; Biswas, M.; Kumar, S.N. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J. Diet. Suppl. 2011, 8, 257–265. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 6), 126–136. [Google Scholar] [CrossRef]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef]
- Prasad, K.; Mantha, S.V.; Muir, A.D.; Westcott, N.D. Protective effect of secoisolariciresinol diglucoside against streptozotocin-induced diabetes and its mechanism. Mol. Cell. Biochem. 2000, 206, 141–149. [Google Scholar] [CrossRef]
- Mason, J.K.; Thompson, L.U. Flaxseed and its lignan and oil components: Can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl. Physiol. Nutr. Metab. 2014, 39, 663–678. [Google Scholar] [CrossRef]
- Calado, A.; Neves, P.M.; Santos, T.; Ravasco, P. The effect of flaxseed in breast cancer: A Literature Review. Front. Nutr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Flower, G.; Fritz, H.; Balneaves, L.G.; Verma, S.; Skidmore, B.; Fernandes, R.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Flax and Breast Cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 181–192. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Kuijsten, A.; Arts, I.C.W.; Vree, T.B.; Hollman, P.C.H. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr. 2005, 135, 795–801. [Google Scholar] [CrossRef]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. Anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gingrich, J.R.; Bao, W.; Li, J.; Haroon, Z.A.; Demark-Wahnefried, W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology 2002, 60, 919–924. [Google Scholar] [CrossRef]
- Chikara, S.; Mamidi, S.; Sreedasyam, A.; Chittem, K.; Pietrofesa, R.; Zuppa, A.; Moorthy, G.; Dyer, N.; Christofidou-Solomidou, M.; Reindl, K.M. Flaxseed consumption inhibits chemically induced lung tumorigenesis and modulates expression of Phase II enzymes and inflammatory cytokines in A/J mice. Cancer Prev. Res. 2018, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed. Cancer Biol. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef]
- Shah, N.R.; Patel, B.M. Secoisolariciresinol diglucoside rich extract of L. usitatissimum prevents diabetic colon cancer through inhibition of CDK4. Biomed. Pharmacother. 2016, 83, 733–739. [Google Scholar] [CrossRef]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and pure secoisolariciresinol diglucoside, but not flaxseed hull, reduce human breast tumor growth (MCF-7) in athymic mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef]
- Edel, A.L.; Patenaude, A.F.; Richard, M.N.; Dibrov, E.; Austria, J.A.; Aukema, H.M.; Pierce, G.N.; Aliani, M. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young healthy adults. Eur. J. Nutr. 2016, 55, 651–663. [Google Scholar] [CrossRef]
- Mason, J.K.; Chen, J.; Thompson, L.U. Flaxseed oil-trastuzumab interaction in breast cancer. Food Chem. Toxicol. 2010, 48, 2223–2226. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar]
- Salem, N., Jr.; Moriguchi, T.; Greiner, R.S.; McBride, K.; Ahmad, A.; Catalan, J.N.; Slotnick, B. Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-fatty acids. J. Mol. Neurosci. 2001, 16, 299–307. [Google Scholar] [CrossRef]
- Lenzi Almeida, K.C.; Teles Boaventura, G.; Guzman Silva, M.A. Influence of omega-3 fatty acids from the flaxseed (Linum usitatissimum) on the brain development of newborn rats. Nutr. Hosp. 2011, 26, 991–996. [Google Scholar]
- Pessanha, C.R.; da Camara Boueri, B.F.; da Costa, L.R.; Ferreira, M.R.; Melo, H.S.; de Abreu, M.D.; Pessoa, L.R.; da Silva, P.C.; Pereira, A.D.; Ribeiro, D.C.; et al. Brain development in male rats subjected to early weaning and treated with diet containing flour or flaxseed oil after 21 days until 60 days. J. Dev. Orig. Health Dis. 2015, 6, 268–271. [Google Scholar] [CrossRef]
- Fernandes, F.S.; de Souza, A.S.; do Carmo, M.; Boaventura, G.T. Maternal intake of flaxseed-based diet (Linum usitatissimum) on hippocampus fatty acid profile: implications for growth, locomotor activity and spatial memory. Nutrition 2011, 27, 1040–1047. [Google Scholar] [CrossRef]
- Mucci Dde, B.; Fernandes, F.S.; Souza Ados, S.; Sardinha, F.L.; Soares-Mota, M.; Tavares do Carmo, M. Flaxseed mitigates brain mass loss, improving motor hyperactivity and spatial memory, in a rodent model of neonatal hypoxic-ischemic encephalopathy. Prostaglandins Leukot. Essent. Fatty Acids 2015, 97, 13–19. [Google Scholar] [CrossRef]
- Naveen, S.; Siddalingaswamy, M.; Singsit, D.; Khanum, F. Anti-depressive effect of polyphenols and omega-3 fatty acid from pomegranate peel and flax seed in mice exposed to chronic mild stress. Psychiatry Clin. Neurosci. 2013, 67, 501–508. [Google Scholar] [CrossRef]
- Ma, X.; Wang, R.; Zhao, X.; Zhang, C.; Sun, J.; Li, J.; Zhang, L.; Shao, T.; Ruan, L.; Chen, L.; et al. Antidepressant-like effect of flaxseed secoisolariciresinol diglycoside in ovariectomized mice subjected to unpredictable chronic stress. Metab. Brain Dis. 2013, 28, 77–84. [Google Scholar] [CrossRef]
- Li, X.B.; Yang, Z.X.; Yang, L.; Chen, X.L.; Zhang, K.; Yang, Q.; Wu, Y.M.; Liu, S.B.; Tao, K.S.; Zhao, M.G. Neuroprotective effects of flax lignan against NMDA-induced neurotoxicity in vitro. CNS Neurosci. Ther. 2012, 18, 927–933. [Google Scholar] [CrossRef]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Munoz, R. Bioactivation of phytoestrogens: Intestinal bacteria and health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef] [PubMed]
- Cetisli, N.E.; Saruhan, A.; Kivcak, B. The effects of flaxseed on menopausal symptoms and quality of life. Holist. Nurs. Pract. 2015, 29, 151–157. [Google Scholar]
- Colli, M.C.; Bracht, A.; Soares, A.A.; de Oliveira, A.L.; Bôer, C.G.; de Souza, C.G.; Peralta, R.M. Evaluation of the efficacy of flaxseed meal and flaxseed extract in reducing menopausal symptoms. J. Med. Food. 2012, 15, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Nickell, L.A.; Thompson, L.U.; Szalai, J.P.; Kiss, A.; Hilditch, J.R. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006, 13, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Dew, T.P.; Williamson, G. Controlled flax interventions for the improvement of menopausal symptoms and postmenopausal bone health: A systematic review. Menopause 2013, 20, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 12, CD001395. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, S.; Qin, R.; Terstreip, S.A.; Liu, H.; Loprinzi, C.L.; Shah, T.R.; Tucker, K.F.; Dakhil, S.R.; Bury, M.J.; Carolla, R.L.; et al. A phase III, randomized, placebo-controlled, double-blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause 2012, 1, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Guarda, D.S.; Lisboa, P.C.; de Oliveira, E.; Nogueira-Neto, J.F.; de Moura, E.G.; Figueiredo, M.S. Flaxseed oil during lactation changes milk and body composition in male and female suckling pups rats. Food Chem. Toxicol. 2014, 69, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Troina, A.A.; Figueiredo, M.S.; Passos, M.C.F.; Reis, A.M.; Oliveira, E.; Lisboa, P.C.; Moura, E.G. Flaxseed bioactive compounds change milk, hormonal and biochemical parameters of dams and offspring during lactation. Food Chem. Toxicol. 2012, 50, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.; Juma, S.; Lucas, E.; Wei, L.; Venkatesh, S.; Khan, D. Flaxseed supplementation positively influences bone metabolism in postmenopausal women. JANA 1998, 1, 27–32. [Google Scholar]
- Kim, Y.; Ilich, J.Z. Implications of dietary α-linolenic acid in bone health. Nutrition 2011, 27, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Neukam, K.; De Spirt, S.; Stahl, W.; Bejot, M.; Maurette, J.M.; Tronnier, H.; Heinrich, U. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin Pharmacol. Physiol. 2011, 24, 67–74. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Aukema, H.M.; Ravandi, A.; Pierce, G.N. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp. Gerontol. 2014, 59, 51–57. [Google Scholar] [CrossRef]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef]
- Thompson, L.U.; Robb, P.; Serraino, M.; Cheung, F. Mammalian lignan production from various foods. Nutr. Cancer 1991, 16, 43–52. [Google Scholar] [CrossRef]
- Wang, C.Z.; Ma, X.Q.; Yang, D.H.; Guo, Z.R.; Liu, G.R.; Zhao, G.X.; Tang, J.; Zhang, Y.N.; Ma, M.; Cai, S.Q.; et al. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010, 10, 115. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef]
- Munoz, O.; Fuentealba, C.; Ampuero, D.; Figuerola, F.; Estévez, A.M. The effect of Lactobacillus acidophilus and Lactobacillus casei on the in vitro bioaccessibility of flaxseed lignans (Linum usitatissimum L.). Food Funct. 2018, 9, 2426–2432. [Google Scholar] [CrossRef]
- Pulkrabek, M.; Rhee, T.; Gibbs, P.; Hall, C. Flaxseed- and Buckwheat-supplemented diets altered enterobacteriaceae diversity and prevalence in the cecum and feces of obese mice. J. Diet. Suppl. 2017, 14, 667–678. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57BI/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Patterson, E.; O’Doherty, R.M.; Murphy, E.F.; Wall, R.; O’Sullivan, O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition on C57BL/6J mice. Br. J. Nutr. 2014, 111, 1905–1917. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yin, P.; Fan, H.; Sun, L.; Liu, Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017, 16, 44. [Google Scholar] [CrossRef]
- Gomides, A.F.; Concalves, R.V.; de Paula, S.O.; Ferreira, C.L.; Comastri, D.S.; Peluzio Mdo, C. Defatted flaxseed meal prevents the appearance of aberrant crypt foci in the colon of mice increasing the gene expression of p53. Nutr. Hosp. 2015, 31, 1675–1681. [Google Scholar]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Blaedel, T.; Håkansson, J.; Dalsgaard, T.K.; Hansen, T.; Pedersen, O.; et al. Dietary modulation of the gut microbiota—A randomized controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114, 406–417. [Google Scholar] [CrossRef]
- van Kranen, H.J.; Mortensen, A.; Sørensen, I.K.; van den Berg-Wijnands, J.; Beems, R.; Nurmi, T.; Adlercreutz, H.; van Kreijl, C.F. Lignan precursors from flaxseed or rye bran do not protect against the development of intestinal neoplasia in ApcMin mice. Nutr.Cancer 2003, 45, 203–210. [Google Scholar] [CrossRef]
- Määttänen, P.; Lurz, E.; Botts, S.R.; Wu, R.Y.; Yeung, C.W.; Li, B.; Abiff, S.; Johnson-Henry, K.C.; Lepp, D.; Power, K.A.; et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G788–G798. [Google Scholar] [CrossRef] [Green Version]
- Soltanian, N.; Janghorbani, M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 29, 41–48. [Google Scholar] [CrossRef]
- Cockerell, K.M.; Watkins, A.S.; Reeves, L.B.; Goddard, L.; Lomer, M.C. Effects of linseeds on the symptoms of irritable bowel syndrome: A pilot randomized controlled trial. J. Hum. Nutr. Diet. 2012, 5, 435–443. [Google Scholar] [CrossRef]
- Hanif, P.A.; Gilani, A.H. Dual effectiveness of flaxseed in constipation and diarrhea: possible mechanism. J. Ethnopharmacol. 2015, 169, 60–68. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Comp. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar]
- Cressey, P.; Reeve, J. Metabolism of cyanogenic glycosides: A review. Food Chem. Toxicol. 2019, 125, 225–232. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051171
Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients. 2019; 11(5):1171. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051171
Chicago/Turabian StyleParikh, Mihir, Thane G. Maddaford, J. Alejandro Austria, Michel Aliani, Thomas Netticadan, and Grant N. Pierce. 2019. "Dietary Flaxseed as a Strategy for Improving Human Health" Nutrients 11, no. 5: 1171. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051171



