Effect of 12-Week Daily Intake of the High-Lycopene Tomato (Solanum Lycopersicum), a Variety Named “PR-7”, on Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Preparation of the Test Food
2.4. Physical, Hematological, Biological, Urinary, and Salivary Assessments
2.5. VAS Questionnaire Assessing Fatigue, Stress, and Profile of Mood States
2.6. Food Frequency Questionnaire
2.7. Assessment of Safety
2.8. Ethics
2.9. Statistical Analysis
2.10. Sample Size
3. Results
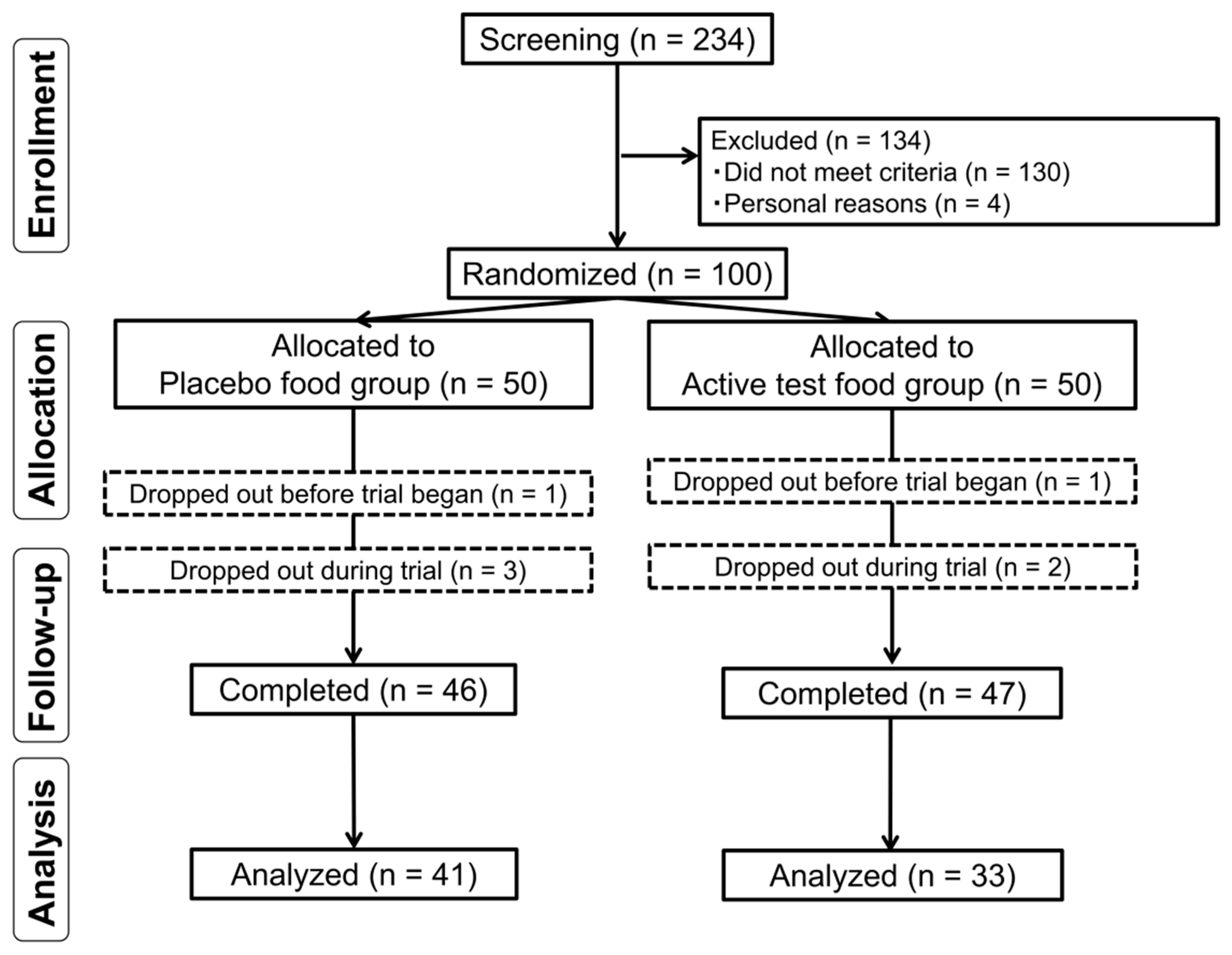
3.1. Subject Dropouts and Characteristics
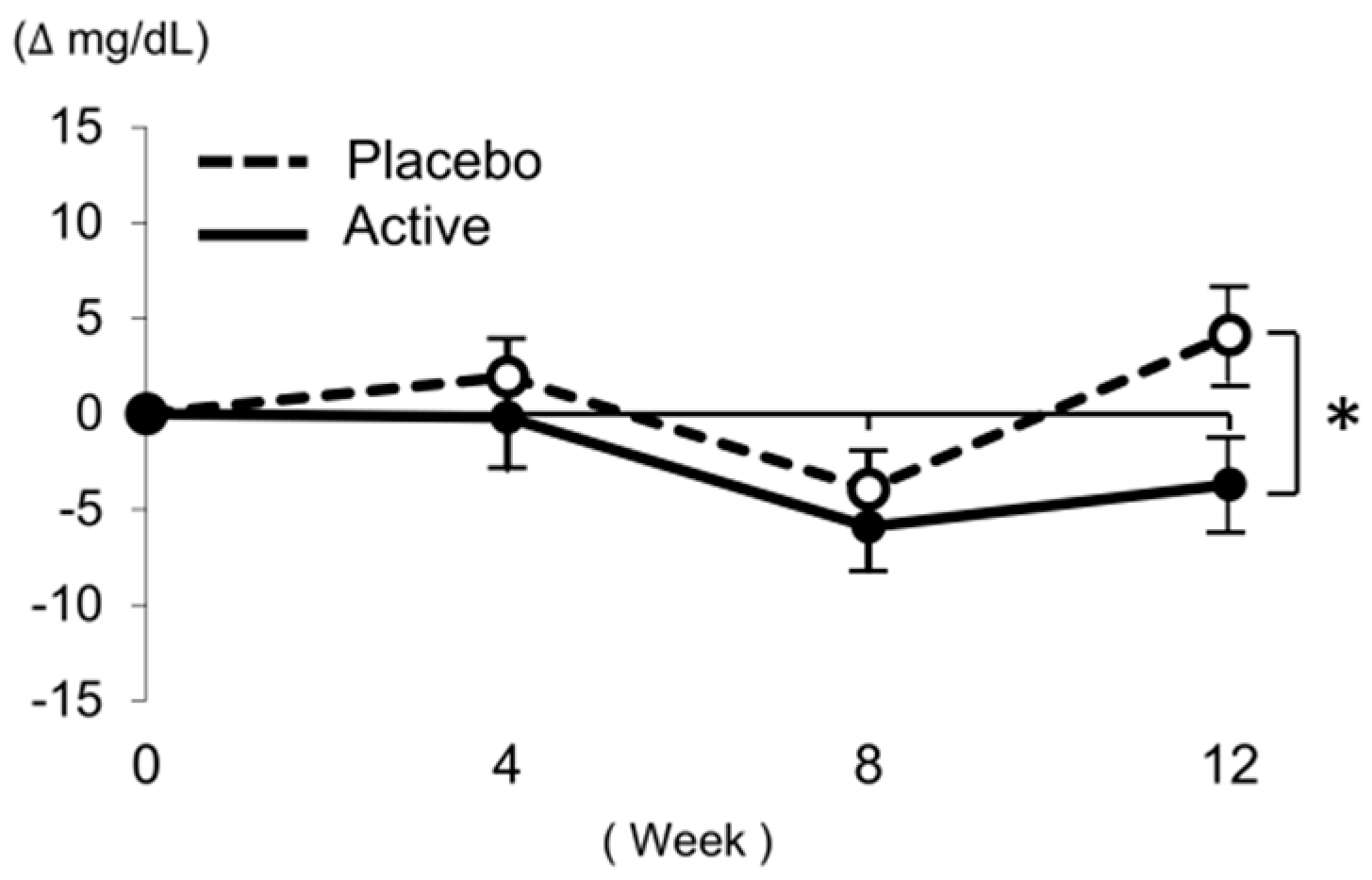
3.2. Effect of High-Lycopene Tomato on LDL-C
3.3. Effect of High-Lycopene Tomato on Lipid Profile and Adiponectin Levels
3.4. Effect of High-Lycopene Tomato on Serum Carotenoid Levels
3.5. Effect of High-Lycopene Tomato on Oxidative Markers
3.6. Effect of High-Lycopene Tomato on Fatigue and Stress
3.7. Assessment of Dietary Nutrients among Subjects during the Study
3.8. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Okamura, T.; Kitamura, A.; Moriyama, Y.; Imano, H.; Sato, S.; Terao, A.; Naito, Y.; Nakagawa, Y.; Kiyama, M.; Tamura, Y.; et al. Plasma level of homocysteine is correlated to extracranial carotid-artery atherosclerosis in non-hypertensive Japanese. J. Cardiovasc. Risk 1999, 6, 371–377. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef]
- Fuhrman, B.; Elis, A.; Aviram, M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 1997, 233, 658–662. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Zhang, L. Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. J. Nutr. Sci. Vitaminol. 2013, 59, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Mancini, B.; Di Ilio, E.; Bucciarelli, T.; D′Orazio, N. Protective effect of lycopene in cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 183–190. [Google Scholar]
- Stice, C.P.; Xia, H.; Wang, X.D. Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development. Chronic. Dis. Transl. Med. 2018, 4, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.J.; Park, S.Y.; Lim, Y.; Kwon, O.; Lee, J.H.; Kim, J.Y. Differential responses of endothelial integrity upon the intake of microencapsulated garlic, tomato extract or a mixture: A single-intake, randomized, double-blind, placebo-controlled crossover trial. Food Funct. 2018, 9, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Paetau, I.; Rao, D.; Wiley, E.R.; Brown, E.D.; Clevidence, B.A. Carotenoids in human buccal mucosa cells after 4 wk of supplementation with tomato juice or lycopene supplements. Am. J. Clin. Nutr. 1999, 70, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Sotoudeh, G.; Ghorbani, M. Tomato juice consumption improves blood antioxidative biomarkers in overweight and obese females. Clin. Nutr. 2015, 34, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microb. 2018, 9, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, S.; Iimuro, S.; Yamashita, H.; Katayama, S.; Ohashi, Y.; Akanuma, Y.; Yamada, N.; Sone, H. Japan Diabetes Complications Study Group, Cohort profile: The Japan diabetes complications study: A long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int. J. Epidemiol. 2014, 43, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Takahashi, A.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; et al. Dietary intake in Japanese patients with type 2 diabetes: Analysis from Japan Diabetes Complications Study. J. Diabetes Investig. 2014, 5, 176–187. [Google Scholar] [CrossRef] [PubMed]
- The criteria for evaluation of adverse reactions and clinical laboratory abnormalities in clinical trials with antimicrobial agents. Chemotherapy 1991, 39, 687–689. Available online: http://www.chemotherapy.or.jp/guideline/39_687.pdf (accessed on 11 April 2019).
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.P.; Royall, D.; Kurian, R.; Muggli, R.; Jeejeebhoy, K.N. Effects of beta-carotene supplementation on lipid peroxidation in humans. Am. J. Clin. Nutr. 1994, 59, 884–890. [Google Scholar] [CrossRef]
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. Measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food Chem. 2011, 59, 3717–3729. [Google Scholar] [CrossRef]
- Hadley, C.W.; Clinton, S.K.; Schwartz, S.J. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J. Nutr. 2003, 133, 727–732. [Google Scholar] [CrossRef]
- Visioli, F.; Riso, P.; Grande, S.; Galli, C.; Porrini, M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur. J. Nutr. 2003, 42, 201–206. [Google Scholar] [CrossRef]
- Bose, K.S.; Agrawal, B.K. Effect of lycopene from cooked tomatoes on serum antioxidant enzymes, lipid peroxidation rate and lipid profile in coronary heart disease. Singapore Med. J. 2007, 48, 415–420. [Google Scholar] [PubMed]
- Briviba, K.; Kulling, S.E.; Moseneder, J.; Watzl, B.; Rechkemmer, G.; Bub, A. Effects of supplementing a low-carotenoid diet with a tomato extract for 2 weeks on endogenous levels of DNA single strand breaks and immune functions in healthy non-smokers and smokers. Carcinogenesis 2004, 25, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.L.; Bernstein, P.S.; Schmidt, M.C.; Von Tress, M.S.; Askew, E.W. Dietary modification and moderate antioxidant supplementation differentially affect serum carotenoids, antioxidant levels and markers of oxidative stress in older humans. J. Nutr. 2003, 133, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Kume, N.; Murase, T.; Minami, M.; Nakagawa, D.; Inada, T.; Tanaka, M.; Ueda, A.; Kominami, G.; Kambara, H.; et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: A novel marker for early diagnosis. Circulation 2005, 112, 812–818. [Google Scholar] [CrossRef]
- Komiyama, M.; Wada, H.; Ono, K.; Yamakage, H.; Satoh-Asahara, N.; Shimada, S.; Akao, M.; Morimoto, T.; Shimatsu, A.; Takahashi, Y.; et al. Smoking cessation reduces the lectin-like low-density lipoprotein receptor index, an independent cardiovascular risk marker of vascular inflammation. Heart Vessels 2018, 33, 9–16. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Fujita, Y.; Kakino, A.; Iwamoto, S.; Takaya, T.; Sawamura, T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc. Drugs Ther. 2011, 25, 379–391. [Google Scholar] [CrossRef]
- Moran, N.E.; Erdman, J.W., Jr.; Clinton, S.K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 2013, 539, 171–180. [Google Scholar] [CrossRef] [Green Version]
| Item | Guidance & Agreement | Screening | Randomization | Test Food-Intake Period | |||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 12 | ||||
| Visit | Visit 1 | – | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
| Date | 20–23 April 2018 | 21 May 2018 | 9–11 June 2018 | 7–9 July 2018 | 4–6 August 2018 | 1–3 September 2018 | |
| Medical interview | ● | ● | ● | ● | ● | ||
| Vital sign measurement | ● | ● | ● | ● | ● | ||
| Body composition measurement | ● | ● | ● | ● | ● | ||
| Blood sampling | ● | ● | ● | ● | ● | ||
| Urinary and salivary test | ● | ● | ● | ● | |||
| VAS questionnaire and POMS-2 | ● | ● | ● | ● | |||
| Food Frequency Questionnaire | ● | ● | ● | ● | |||
| Inclusion criteria | 1. Age, ≥30 years and <70 years old |
| 2. LDL-C, ≥120 mg/dL and <160 mg/dL | |
| Main exclusion criteria | 1. Subjects who participated in the pilot study |
| 2. Subjects who usually do not consume raw tomatoes | |
| 3. Subjects who usually consume tomato juice | |
| 4. Subjects under physician’s advice, treatment, and/or medication for dyslipidemia and/or diabetes | |
| 5. Subjects with a BMI ≥30 kg/m2 | |
| 6. Subjects with familial hypercholesterolemia | |
| 7. Subjects with serious cerebrovascular, cardiac, hepatic, renal, gastrointestinal diseases, and/or affected by infectious diseases requiring reports to the authorities | |
| 8. Subjects with a major surgical history relevant to the digestive system, such as gastrectomy, gastrorrhaphy, enterectomy, etc. | |
| 9. Subjects with unusually high and/or low blood pressure and/or abnormal hematological data | |
| 10. Subjects with severe anemia | |
| 11. Pre- or post-menopausal women complaining of obvious physical changes | |
| 12. Subjects at risk of allergic reactions to drugs or foods especially due to tomato, Japanese cedar, Japanese cypress, or grass | |
| 13. Subjects who regularly take medications, functional foods, and/or supplements, which would affect blood lipid and/or glucose metabolism | |
| 14. Alcohol addicts or subjects with an eating disorder | |
| 15. Subjects who donated either 400 mL of whole blood within 16 weeks (women) or 12 weeks (men), 200 mL of whole blood within 4 weeks (men and women), or blood components within 2 weeks (men and women) prior to the current study. | |
| 16. Pregnant or lactating women or women who expect to be pregnant during this study | |
| 17. Subjects who currently participate in other clinical trials or have participated in a trial within the last 4 weeks prior to the current study | |
| 18. Any other medical and/or health reasons unfavorable to participation in the current study, as judged by the principal investigator |
| Nutrient | Active Test Food | Placebo Food |
|---|---|---|
| Calories (kcal) | 57.5 | 45.7 |
| Water (g) | 33.2 | 36.5 |
| Proteins (g) | 2.1 | 2.1 |
| Lipids (g) | 0.4 | 0.4 |
| Carbohydrates (g) | 12.8 | 9.8 |
| Ash (g) | 1.7 | 1.2 |
| Total fiber (g) | 2.6 | 2.6 |
| Sodium (mg) | 3.1 | 2.3 |
| Lycopene (mg) | 22.0–27.8 | n.d. |
| β-Carotene (mg) | 2.8-3.3 | n.d. |
| Characteristic | Placebo | Active | p |
|---|---|---|---|
| Subjects, n | 41 | 33 | - |
| Male, n | 17 | 8 | 0.14 |
| Age, years | 53.9 ± 9.1 | 53.7 ± 7.8 | 0.91 |
| Height, cm | 162.7 ± 8.8 | 160.4 ± 8.3 | 0.27 |
| Body mass index, kg/m2 | 21.7 ± 2.8 | 22.1 ± 3.0 | 0.59 |
| LDL cholesterol, mg/dL | 137.3 ± 12.4 | 139.2 ± 12.5 | 0.51 |
| Intake rate, % | 99.8 ± 0.5 | 99.9 ± 0.4 | 0.45 |
| Variable | Week 0 | ΔWeek 4 | ΔWeek 8 | ΔWeek 12 | Time × Food Interaction, p b | |
|---|---|---|---|---|---|---|
| LDL-C (mg/dL) | Placebo (n = 41) | 133.4 ± 15.6 | 1.9 ± 12.1 | −4.0 ± 12.4 | 4.1 ± 15.7 | 0.13 |
| Active (n = 33) | 140.2 ± 16.9 | −0.2 ± 15.5 | −5.9 ± 13.0 | −3.7 ± 13.8 | ||
| pa | 0.078 | 0.514 | 0.511 | 0.027 * | ||
| LDL-C subjects whose LDL-C was 120–139 mg/dL (mg/dL) | Placebo (n = 41) | 122.1±10.1 | 1.8±8.5 | -2.0±10.6 | 4.3±15.1 | 0.048 * |
| Active (n = 33) | 127.8±10.1 | 3.2±11.9 | -6.2±7.8 | -5.1±9.5 | ||
| pa | 0.100 | 0.686 | 0.180 | 0.030 * | ||
| LDL-C subjects whose LDL-C was 140–159 mg/dL (mg/dL) | Placebo (n = 41) | 143.2 ± 12.6 | 2.0 ± 14.9 | −5.7 ± 13.9 | 4.0 ± 16.6 | 0.354 |
| Active (n = 33) | 153.4 ± 11.8 | −3.9 ± 18.3 | −5.6 ± 17.2 | −2.2 ± 17.5 | ||
| pa | 0.016 * | 0.286 | 0.981 | 0.276 | ||
| TC (mg/dL) | Placebo (n = 41) | 222.5 ± 23.9 | 0.9 ± 15.1 | −1.5 ± 18.0 | 7.9 ± 21.7 | 0.20 |
| Active (n = 33) | 231.8 ± 24.6 | −1.6 ± 18.5 | −1.3 ± 17.0 | 1.2 ± 19.1 | ||
| pa | 0.10 | 0.51 | 0.96 | 0.17 | ||
| HDL-C (mg/dL) | Placebo (n = 41) | 75.2 ± 17.5 | −1.0 ± 5.8 | −1.9 ± 7.5 | −0.6 ± 8.4 | 0.76 |
| Active (n = 33) | 80.7 ± 17.4 | −3.0 ± 7.3 | −3.0 ± 6.5 | −2.7 ± 7.8 | ||
| pa | 0.18 | 0.21 | 0.49 | 0.27 | ||
| TG (mg/dL) | Placebo (n = 41) | 86.6 ± 30.7 | −2.4±25.5 | −3.7 ± 28.5 | 0.4 ± 24.8 | 0.83 |
| Active (n = 33) | 80.8 ± 32.8 | −3.2±24.1 | −0.9 ± 20.6 | 3.2 ± 31.2 | ||
| pa | 0.43 | 0.89 | 0.64 | 0.66 | ||
| LDL-C/HDL-C ratio | Placebo (n = 41) | 1.9 ± 0.4 | 0.1 ± 0.2 | 0.0 ± 0.2 | 0.1 ± 0.2 | 0.24 |
| Active (n = 33) | 1.8 ± 0.4 | 0.1 ± 0.2 | 0.0 ± 0.2 | 0.0 ± 0.2 | ||
| pa | 0.67 | 0.67 | 0.72 | 0.33 | ||
| non-HDL (mg/dL) | Placebo (n = 41) | 147.3 ± 15.9 | 1.9 ± 14.0 | 0.4 ± 14.1 | 8.5 ± 17.0 | 0.20 |
| Active (n = 33) | 151.2 ± 16.9 | 1.4 ± 14.1 | 1.8 ± 13.2 | 3.9 ± 15.6 | ||
| pa | 0.32 | 0.87 | 0.67 | 0.24 | ||
| Adiponectin (μg/mL) | Placebo (n = 41) | 10.9 ± 5.9 | −0.3 ± 1.1 | −0.6 ± 1.3 | −0.5 ± 1.2 | 0.005 ** |
| Active (n = 33) | 13.6 ± 7.1 | 0.1 ± 1.1 | −0.6 ± 1.4 | −1.0 ± 1.0 | ||
| pa | 0.085 | 0.17 | 0.97 | 0.053 |
| Variable | Week 0 | ΔWeek 4 | ΔWeek 8 | ΔWeek 12 | Time × Food Interaction, p b | |
|---|---|---|---|---|---|---|
| Lycopene (μg/dL) | Placebo (n = 41) | 75.2 ± 45.9 | −26.1 ± 36.2 | −20.5 ± 47.8 | −24.2 ± 49.3 | 0.64 |
| Active (n = 33) | 85.9 ± 53.0 | 14.1 ± 49.0 | 25.4 ± 42.0 | 22.7 ± 47.9 | ||
| pa | 0.36 | p < 0.001 ** | p < 0.001 ** | p < 0.001 ** | ||
| β-carotene (μg/dL) | Placebo (n = 41) | 37.6 ± 29.2 | −2.2 ± 10.6 | −0.3 ± 13.9 | −0.9 ± 13.6 | 0.45 |
| Active (n = 33) | 51.8 ± 45 | 10.9 ± 20.9 | 16.2 ± 25.1 | 12.0 ± 24.5 | ||
| pa | 0.13 | 0.002 ** | 0.001 ** | 0.009 ** |
| Variable | Week 0 | ΔWeek 4 | ΔWeek 8 | ΔWeek 12 | Time × Food Interaction, p b | |
|---|---|---|---|---|---|---|
| MDA-LDL (U/L) | Placebo (n = 41) | 156.6 ± 47.5 | −45.6 ± 49.5 | −19.5 ± 47.9 | 6.8 ± 54.5 | 0.94 |
| Active (n = 33) | 151.5 ± 45.2 | −41.3 ± 32.7 | −14.9 ± 48.7 | 7.8 ± 46.9 | ||
| pa | 0.65 | 0.65 | 0.68 | 0.94 | ||
| LOX-index | Placebo (n = 41) | 459.7 ± 514.9 | −141.2 ± 299.2 | −43.8 ± 287.2 | 698.5 ± 3527.1 | 0.41 |
| Active (n = 33) | 303.1 ± 118.4 | −40.8 ± 165.7 | 45.2 ± 250.3 | 278.8 ± 311.4 | ||
| pa | 0.066 | 0.089 | 0.17 | 0.50 | ||
| sLOX-1 (pg/mL) | Placebo (n = 41) | 472.3 ± 511.1 | 11.3 ± 243.2 | 19.3 ± 176.2 | 675.0 ± 3688.7 | 0.39 |
| Active (n = 33) | 291.7 ± 127.1 | 127.6 ± 333.5 | 103.9 ± 196.3 | 226.0 ± 242.1 | ||
| pa | 0.034 * | 0.087 | 0.055 | 0.49 | ||
| LAB (μg·cs/mL) | Placebo (n = 41) | 1.1 ± 0.3 | −0.4 ± 0.3 | −0.2 ± 0.3 | 0.0 ± 0.3 | 0.23 |
| Active (n = 33) | 1.1 ± 0.2 | −0.4 ± 0.3 | −0.2 ± 0.3 | 0.1 ± 0.3 | ||
| pa | 0.67 | 0.63 | 0.63 | 0.48 | ||
| LPO (nmol/mL) | Placebo (n = 41) | 4.2 ± 0.6 | −1.1 ± 0.6 | −0.7 ± 0.6 | −0.5 ± 0.6 | 0.34 |
| Active (n = 33) | 4.1 ± 0.5 | −1.2 ± 0.6 | −0.7 ± 0.5 | −0.5 ± 0.4 | ||
| pa | 0.83 | 0.32 | 0.87 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, M.; Tominaga, N.; Ishikawa-Takano, Y.; Maeda-Yamamoto, M.; Nishihira, J. Effect of 12-Week Daily Intake of the High-Lycopene Tomato (Solanum Lycopersicum), a Variety Named “PR-7”, on Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 11, 1177. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051177
Nishimura M, Tominaga N, Ishikawa-Takano Y, Maeda-Yamamoto M, Nishihira J. Effect of 12-Week Daily Intake of the High-Lycopene Tomato (Solanum Lycopersicum), a Variety Named “PR-7”, on Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients. 2019; 11(5):1177. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051177
Chicago/Turabian StyleNishimura, Mie, Naoki Tominaga, Yuko Ishikawa-Takano, Mari Maeda-Yamamoto, and Jun Nishihira. 2019. "Effect of 12-Week Daily Intake of the High-Lycopene Tomato (Solanum Lycopersicum), a Variety Named “PR-7”, on Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study" Nutrients 11, no. 5: 1177. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051177