Overcoming the Bitter Taste of Oils Enriched in Fatty Acids to Obtain Their Effects on the Heart in Health and Disease
Abstract
:1. The Challenge of Incorporating Fatty Acid into the Diet
2. Introduction to the Biological Activity of Fatty Acids
3. Dietary Fatty Acids and Cardiovascular Disease
4. Polyunsaturated Fatty Acids in Atherosclerosis
5. Fatty Acids and Ischemic Heart Disease
6. Fatty Acid Metabolism in the Heart in Obesity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
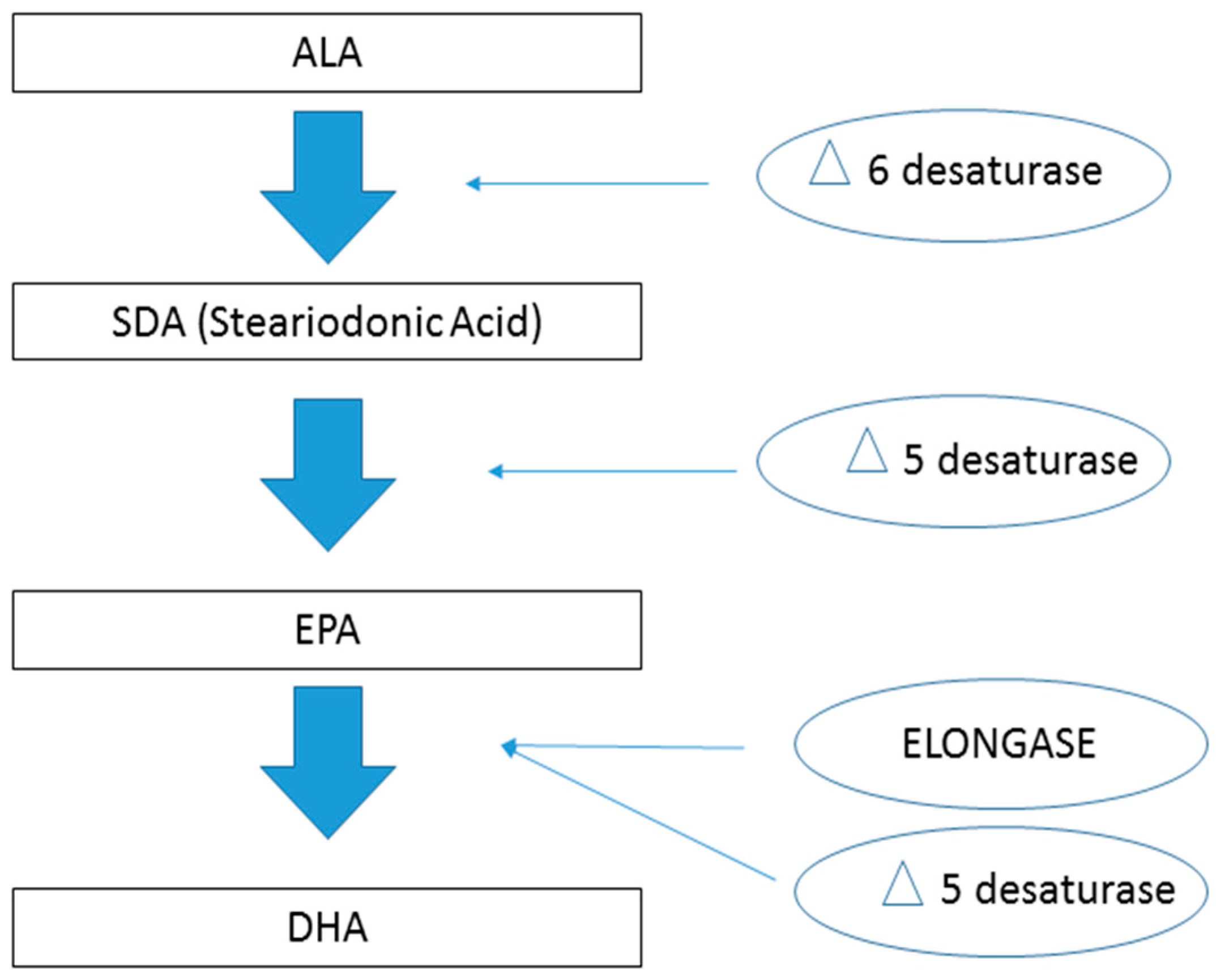
References
- Busch, J.L.; Hrncirik, K.; Bulukin, E.; Boucon, C.; Mascini, M. Biosensor measurements of polar phenolics for the assessment of the bitterness and pungency of virgin olive oil. J. Agric. Food Chem. 2006, 54, 4371–4377. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.W.; Mackenzie, K.; Vincent, W.; Krokhin, O.V. Characterization and complete separation of major cyclolinopeptides in flaxseed oil by reversed-phase chromatography. J. Sep. Sci. 2014, 37, 1788–1796. [Google Scholar] [CrossRef]
- Vitaglione, P.; Savarese, M.; Paduano, A.; Scalfi, L.; Fogliano, V.; Sacchi, R. Healthy virgin olive oil: A matter of bitterness. Crit. Rev. Food Sci. Nutr. 2015, 55, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.A.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavoured virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L.; Matthäus, B.; Fehling, E.; Wiege, B.; Lehmann, B.; Luftmann, H.; Bergander, K.; Quiroga, K.; Scheipers, A.; Frank, O.; et al. Identification of bitter off-taste compounds in the stored cold pressed linseed oil. J. Agric. Food Chem. 2007, 55, 7864–7868. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Adhikari, K.; Chambers, E., 4th; Chambers, D.H.; Carbonell-Barrachina, A.A. Cross-cultural perception of six commercial olive oils: A study with Spanish and US consumers. Food Sci. Technol. Int. 2015, 21, 454–466. [Google Scholar] [CrossRef]
- Aliani, M.; Tyland, D.; Pierce, G.N. Effect of flax addition on the flavor profile of muffins and snack bars. Food Res. Int. 2011, 44, 2489–2496. [Google Scholar] [CrossRef]
- Yousfi, K.; Cayuela, J.A.; García, J.M. Reduction of virgin olive oil bitterness by fruit cold storage. J. Agric. Food Chem. 2008, 56, 10085–10091. [Google Scholar] [CrossRef]
- García, J.M.; Yousfi, K.; Mateos, R.; Olmo, M.; Cert, A. Reduction of oil bitterness by heating of olive (Olea europaea) fruits. J. Agric. Food Chem. 2001, 49, 4231–4235. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Škevin, D.; Petričević, S.; Brkić Bubola, K.; Mokrovčak, Ž. Bitterness, odor properties and volatile compounds of virgin olive oil with phospholipids addition. LWT-Food Sci. Technol. 2009, 42, 50–55. [Google Scholar] [CrossRef]
- Abenoza, M.; Raso, J.; Oria, R.; Sánchez-Gimeno, A.C. Modulating the bitterness of Empeltre olive oil by partitioning polyphenols between oil and water phases: Effect on quality and shelf life. Food Sci. Technol. Int. 2019, 25, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Austria, J.A.; Aliani, M.; Malcolmson, L.J.; Dibrov, E.; Blackwood, E.P.; Maddaford, T.G.; Guzman, R.; Pierce, G.N. Daily food choices over one year when patient diets are supplemented with milled flaxseed. J. Funct. Foods 2016, 26, 772–780. [Google Scholar] [CrossRef]
- Austria, J.A.; Richard, M.N.; Chahine, M.N.; Edel, A.L.; Malcolmson, L.J.; Dupasquier, C.M.C.; Pierce, G.N. Bioavailability of alpha linolenic acid in subjects after ingestion of three different forms of flaxseed. J. Am. Coll. Nutr. 2008, 27, 214–221. [Google Scholar] [CrossRef]
- Kaul, N.; Kreml, R.; Austria, J.A.; Landry, M.N.; Edel, A.L.; Dibrov, E.; Hirono, S.; Zettler, M.E.; Pierce, G.N. A comparative effect of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J. Am. Coll. Nutr. 2008, 27, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- An, D.; Pulinilkunnil, T.; Qi, D.; Ghosh, S.; Abrahani, A.; Rodrigues, B. The metabolic “switch” AMPK regulates cardiac heparin-releasable lipoprotein lipase. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E246–E253. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Ly, L.H.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol. 2004, 173, 6151–6160. [Google Scholar] [CrossRef]
- Wong, S.W.; Kwon, M.J.; Choi, A.M.; Kim, H.P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef]
- Tribulova, N.; Szeiffova Bacova, B.; Egan Benova, T.; Knezl, V.; Barancik, M.; Slezak, J. Omega-3 index and anti-arrhythmic potential of omega-3 PUFAs. Nutrients 2017, 9, 1191. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef] [Green Version]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bosse, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef]
- Wu, K.K.; Frasier-Scott, K.; Hatzakis, H. Endothelial cell function in hemostasis and thrombosis. Adv. Exp. Med. Biol. 1988, 242, 127–133. [Google Scholar]
- Stamenkovic, A.; Pierce, G.N.; Ravandi, A. Oxidized phosphatidylcholine: Not just another brick in the wall. Can. J. Physiol. Pharmacol. 2018, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, A.; O’Hara, K.A.; Nelson, D.C.; Maddaford, T.G.; Edel, A.L.; Maddaford, G.G.; Dibrov, E.; Aghanoori, M.R.; Fernyhough, P.; Kirshenbaum, L.A.; et al. Oxidized phosphatidylcholines cause ferroptosis in cardiomyocytes during ischemia/reperfusion injury. 2019; in press. [Google Scholar]
- Stamenkovic, A.; Pierce, G.N.; Ravandi, A. Phospholipid oxidation products in ferroptotic myocardial cell death. Am. J. Physiol. 2019, in press. [Google Scholar] [CrossRef]
- Adler, D.H.; Cogan, J.D.; Phillips, J.A., 3rd; Schnetz-Boutaud, N.; Milne, G.L.; Iverson, T.; Stein, J.A.; Brenner, D.A.; Morrow, J.D.; Boutaud, O.; et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J. Clin. Investig. 2008, 118, 2121–2131. [Google Scholar] [PubMed]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Michas, G.; Micha, R.; Zampelas, A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 2014, 234, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Willett, W.C. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J. Cardiovasc. Med. 2007, 8 (Suppl. 1), S42–S45. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Greenwood, M.R.C.; New York Academy of Sciences. Foods for Health in the 21st Century: A Roadmap for the Future; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bassett, C.M.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.; Pierce, G.N. Dietary vaccenic acid has antiatherogenic effects in LDLr-/- mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef]
- Bassett, C.M.; McCullough, R.S.; Edel, A.L.; Maddaford, T.G.; Dibrov, E.; Blackwood, D.P.; Austria, J.A.; Pierce, G.N. Trans-fatty acids in the diet stimulate atherosclerosis. Metabolism 2009, 58, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.; McCullough, R.S.; Edel, A.L.; Patenaude, A.; LaVallee, R.K.; Pierce, G.N. The alpha-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2220–H2226. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Lytwyn, M.S.; Pierce, G.N. Differential effects of trans and polyunsaturated fatty acids on ischemia/reperfusion injury and its associated cardiovascular disease states. Curr. Pharm. Des. 2013, 19, 6858–6863. [Google Scholar] [CrossRef]
- Hadj Ahmed, S.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Najjar, M.F.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef]
- Hadj Ahmed, S.; Kaoubaa, N.; Kharroubi, W.; Zarrouk, A.; Najjar, M.F.; Batbout, F.; Gamra, H.; Lizard, G.; Hammami, M. Association of plasma fatty acid alteration with the severity of coronary artery disease lesions in Tunisian patients. Lipids Health Dis. 2017, 16, 154. [Google Scholar] [CrossRef]
- Bourne, G.H. Aspects of Human Nutrition; Karger: New York, NY, USA, 1988. [Google Scholar]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Balter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.C.; Jennings, R.B. Myocardial calcium and magnesium in acute ischemic injury. Am. J. Pathol. 1972, 67, 417–440. [Google Scholar] [PubMed]
- Ander, B.P.; Hurtado, C.; Raposo, C.S.; Maddaford, T.G.; Deniset, J.F.; Hryshko, L.V.; Pierce, G.N.; Lukas, A. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc. Res. 2007, 73, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Pankow, J.S.; Eckfeldt, J.H.; Folsom, A.R.; Hopkins, P.N.; Province, M.A.; Hong, Y.; Ellison, R.C. Relation between dietary linolenic acid and coronary artery disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Clin. Nutr. 2001, 74, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef]
- Mensink, R.P. Metabolic and health effects of isomeric fatty acids. Curr. Opin. Lipidol. 2005, 16, 27–30. [Google Scholar] [CrossRef]
- Ganguly, R.; Pierce, G.N. Transfat involvement in cardiovascular disease. Mol. Nutr. Food Res. 2012, 56, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Chardigny, J.M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 2), S5–S21. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Sacks, F.M.; Salvini, S.; Willett, W.C.; Hennekens, C.H. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 1991, 325, 373–381. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- Ascherio, A.; Katan, M.B.; Zock, P.L.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and coronary heart disease. N. Engl. J. Med. 1999, 340, 1994–1998. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Meigs, J.B.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005, 135, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.H.; Smialek, J.; Virmani, R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Shinke, T.; Toh, R.; Ishida, T.; Otake, H.; Takaya, T.; Sugiyama, D.; Toba, T.; Kuroda, M.; Takahashi, H.; et al. The impact of serum trans fatty acids concentration on plaque vulnerability in patients with coronary artery disease: Assessment via optical coherence tomography. Atherosclerosis 2017, 265, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, A.H. Dietary trans fatty acids and cardiovascular disease risk: Past and present. Curr. Atheroscler. Rep. 2014, 16, 433. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. The seven countries study: 2289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Prospective Urban Rural Epidemiology study. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Dietary alpha-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef]
- Massiera, F.; Barbry, P.; Guesnet, P.; Joly, A.; Luquet, S.; Moreilhon-Brest, C.; Mohsen-Kanson, T.; Amri, E.Z.; Ailhaud, G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 2006, 51, 2352–2361. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; Telis, N.; German, J.B.; Hammock, B.D. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif. Agric. (Berkeley) 2011, 65, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Takayama, K.; Yuhki, K.; Ono, K.; Fujino, T.; Hara, A.; Yamada, T.; Kuriyama, S.; Karibe, H.; Okada, Y.; Takahata, O.; et al. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat. Med. 2006, 11, 562–566. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Zenebe, W.J.; Parihar, A.; Parihar, M.S.; Vaccaro, M.; Rink, C.; Sen, C.K.; Ghafourifar, P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch. Biochem. Biophys. 2007, 468, 114–120. [Google Scholar] [CrossRef]
- Hall, D.W.; Jaitly, K.D. Structure-activity relationships in a series of 11-deoxy prostaglandins. Prostaglandins 1976, 11, 573–587. [Google Scholar] [CrossRef]
- Geoffroy, J.; Benzoni, D.; Sassard, J. Antihypertensive effect of thromboxane A2 receptor blockage in genetically hypertensive rats of the Lyon strain. J. Hypertens. Suppl. 1989, 7, S272–S273. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Hercule, H.C.; Schunck, W.H.; Gross, V.; Seringer, J.; Leung, F.P.; Weldon, S.M.; da Costa Goncalves, A.; Huang, Y.; Luft, F.C.; Gollasch, M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Mozaffarian, D.; Kuller, L.H.; Tracy, R.P.; Siscovick, D.S. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2003, 77, 319–325. [Google Scholar] [CrossRef]
- Steering Committee of the Physicians’ Health Study Research Group. Preliminary report: Findings from the aspirin component of the ongoing Physicians’ Health Study. N. Engl. J. Med. 1988, 318, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Konno, S.; Tamura, K.; Kimura, B.; Kawano, K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke 1992, 23, 1400–1403. [Google Scholar] [CrossRef]
- van Diemen, J.J.; Fuijkschot, W.W.; Wessels, T.J.; Veen, G.; Smulders, Y.M.; Thijs, A. Evening intake of aspirin is associated with a more stable 24-h platelet inhibition compared to morning intake: A study in chronic aspirin users. Platelets 2016, 27, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, V.; Busse, R.; Fleming, I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension 2003, 41 Pt 2, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.A.; Balazy, M.; Margiotta, P.; Huang, D.D.; Falck, J.R.; McGiff, J.C. Cytochrome P-450-dependent HETEs: Profile of biological activity and stimulation by vasoactive peptides. Am. J. Physiol. 2003, 271 Pt 2, R863–R869. [Google Scholar] [CrossRef]
- Fang, X.; Faraci, F.M.; Kaduce, T.L.; Harmon, S.; Modrick, M.L.; Hu, S.; Moore, S.A.; Falck, J.R.; Weintraub, N.L.; Spector, A.A. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: Role of cyclooxygenase. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2301–H2307. [Google Scholar] [CrossRef]
- Back, M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Sato, M.; Yokoyama, U.; Fujita, T.; Okumura, S.; Ishikawa, Y. The roles of cytochrome p450 in ischemic heart disease. Curr. Drug Metab. 2011, 12, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Zeldin, D.C.; Hoffman, S.M.; Maltais, L.J.; Wain, H.M.; Nebert, D.W. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 2004, 14, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuchardt, J.P.; Schmidt, S.; Kressel, G.; Dong, H.; Willenberg, I.; Hammock, B.D.; Hahn, A.; Schebb, N.H. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Chen, W.; Murphy, E.; Gabel, S.; Tomer, K.B.; Foley, J.; Steenbergen, C.; Falck, J.R.; Moomaw, C.R.; Zeldin, D.C. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J. Biol. Chem. 1997, 272, 12551–12559. [Google Scholar] [CrossRef] [PubMed]
- Batchu, S.N.; Law, E.; Brocks, D.R.; Falck, J.R.; Seubert, J.M. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J. Mol. Cell Cardiol. 2009, 46, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Seubert, J.M.; Zeldin, D.C.; Nithipatikom, K.; Gross, G.J. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Lipid Mediat. 2007, 82, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shureiqi, I.; Wojno, K.J.; Poore, J.A.; Reddy, R.G.; Moussalli, M.J.; Spindler, S.A.; Greenson, J.K.; Normolle, D.; Hasan, A.A.; Lawrence, T.S.; et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis 1999, 20, 1985–1995. [Google Scholar] [CrossRef]
- Hawcroft, G.; Loadman, P.M.; Belluzzi, A.; Hull, M.A. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signaling in human colorectal cancer cells. Neoplasia 2010, 12, 618–627. [Google Scholar] [CrossRef]
- Kramer, H.J.; Stevens, J.; Grimminger, F.; Seeger, W. Fish oil fatty acids and human platelets: Dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem. Pharmacol. 1996, 52, 1211–1217. [Google Scholar] [CrossRef]
- Needleman, P.; Raz, A.; Minkes, M.S.; Ferrendelli, J.A.; Sprecher, H. Triene prostaglandins: Prostacyclin and thromboxane biosynthesis and unique biological properties. Proc. Natl. Acad. Sci. USA 1979, 76, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.A.; Deniset, J.F.; Austria, J.A.; LaVallee, R.K.; Maddaford, G.G.; Hedley, T.E.; Dibrov, E.; Pierce, G.N. Effects of dietary flaxseed on atherosclerotic plaque regression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1743–H1751. [Google Scholar] [CrossRef] [Green Version]
- Westphal, C.; Konkel, A.; Schunck, W.H. CYP-eicosanoids—A new link between omega-3 fatty acids and cardiac disease? Prostaglandins Lipid Mediat. 2011, 96, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koh, H.W.; Choi, H.; Koh, W.P.; Yuan, J.M.; Newman, J.W.; Su, J.; Fang, J.; Ong, C.N.; van Dam, R.M. Plasma fatty acids, oxylipins, and risk of myocardial infarction: The Singapore Chinese Health Study. J. Lipid Res. 2016, 57, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Botham, K.M.; Bravo, E.; Elliott, J.; Wheeler-Jones, C.P. Direct interaction of dietary lipids carried in chylomicron remnants with cells of the artery wall: Implications for atherosclerosis development. Curr. Pharm. Des. 2005, 11, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.C.; Mamo, J.C. Chylomicron-remnant-induced foam cell formation and cytotoxicity: A possible mechanism of cell death in atherosclerosis. Clin. Sci. (Lond.) 2000, 98, 183–192. [Google Scholar] [CrossRef]
- De Pascale, C.; Avella, M.; Perona, J.S.; Ruiz-Gutierrez, V.; Wheeler-Jones, C.P.; Botham, K.M. Fatty acid composition of chylomicron remnant-like particles influences their uptake and induction of lipid accumulation in macrophages. FEBS J. 2006, 273, 5632–5640. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Madonna, R.; Massaro, M. Effects of omega-3 fatty acids on cytokines and adhesion molecules. Curr. Atheroscler. Rep. 2004, 6, 485–491. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomized controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic Modulators in Heart Disease: Past, Present, and Future. Can. J. Cardiol. 2017, 33, 838–849. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Mommsen, T.P. Protons and anaerobiosis. Science 1983, 219, 1391–1397. [Google Scholar] [CrossRef]
- Liu, B.; Clanachan, A.S.; Schulz, R.; Lopaschuk, G.D. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ. Res. 1996, 79, 940–948. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Barr, R.; Thomas, P.D.; Dyck, J.R. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ. Res. 2003, 93, e33–e37. [Google Scholar] [CrossRef]
- Ganguly, R.; Hasanally, D.; Stamenkovic, A.; Maddaford, T.G.; Chaudhary, R.; Pierce, G.N.; Ravandi, A. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol. Cell. Biochem. 2018, 437, 163–175. [Google Scholar] [CrossRef]
- White, C.W.; Hasanally, D.; Mundt, P.; Li, Y.; Xiang, B.; Klein, J.; Muller, A.; Ambrose, E.; Ravandi, A.; Arora, R.C.; et al. A whole blood-based perfusate provides superior preservation of myocardial function during ex vivo heart perfusion. J. Heart Lung Transplant. 2015, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Hasanally, D.; Que, X.; Hung, M.Y.; Stamenkovic, A.; Chan, D.; Chaudhary, R.; Margulets, V.; Edel, A.L.; Hoshijima, M.; et al. Reduction of myocardial ischemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2019, 115, 179–189. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Lyall, D.M.; Gray, S.R.; Steell, L.; Anderson, J.; Iliodromiti, S.; Welsh, P.; Guo, Y.; Petermann, F.; Mackay, D.F.; et al. Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: Evidence from 48 170 UK Biobank participants. Int. J. Obes. (Lond.) 2017, 41, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Mirzaei, F.; O’Reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef]
- Javadi, M.; Geelen, M.J.; Everts, H.; Hovenier, R.; Javadi, S.; Kappert, H.; Beynen, A.C. Effect of dietary conjugated linoleic acid on body composition and energy balance in broiler chickens. Br. J. Nutr. 2007, 98, 1152–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlhausler, B.S.; Ailhaud, G.P. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Keung, W.; Samokhvalov, V.; Wang, W.; Lopaschuk, G.D. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim. Biophys. Acta 2010, 1801, 1–22. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc. Res. 2008, 79, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonen, A.; Campbell, S.E.; Benton, C.R.; Chabowski, A.; Coort, S.L.; Han, X.X.; Koonen, D.P.; Glatz, J.F.; Luiken, J.J. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc. Nutr. Soc. 2004, 63, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahey, R.; Wang, X.; Carley, A.N.; Lewandowski, E.D. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation 2014, 130, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Lehto, H.R.; Parkka, J.; Borra, R.; Tuunanen, H.; Lepomaki, V.; Parkkola, R.; Knuuti, J.; Nuutila, P.; Iozzo, P. Effects of acute and one-week fatty acid lowering on cardiac function and insulin sensitivity in relation with myocardial and muscle fat and adiponectin levels. J. Clin. Endocrinol. Metab. 2012, 97, 3277–3284. [Google Scholar] [CrossRef]
- Wolf, P.; Winhofer, Y.; Krssak, M.; Smajis, S.; Harreiter, J.; Kosi-Trebotic, L.; Furnsinn, C.; Anderwald, C.H.; Baumgartner-Parzer, S.; Trattnig, S.; et al. Suppression of plasma free fatty acids reduces myocardial lipid content and systolic function in type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 387–392. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamenkovic, A.; Ganguly, R.; Aliani, M.; Ravandi, A.; Pierce, G.N. Overcoming the Bitter Taste of Oils Enriched in Fatty Acids to Obtain Their Effects on the Heart in Health and Disease. Nutrients 2019, 11, 1179. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051179
Stamenkovic A, Ganguly R, Aliani M, Ravandi A, Pierce GN. Overcoming the Bitter Taste of Oils Enriched in Fatty Acids to Obtain Their Effects on the Heart in Health and Disease. Nutrients. 2019; 11(5):1179. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051179
Chicago/Turabian StyleStamenkovic, Aleksandra, Riya Ganguly, Michel Aliani, Amir Ravandi, and Grant N. Pierce. 2019. "Overcoming the Bitter Taste of Oils Enriched in Fatty Acids to Obtain Their Effects on the Heart in Health and Disease" Nutrients 11, no. 5: 1179. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051179




