Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Antibodies
2.2. Isolation and Culturing of Human Chondrocytes
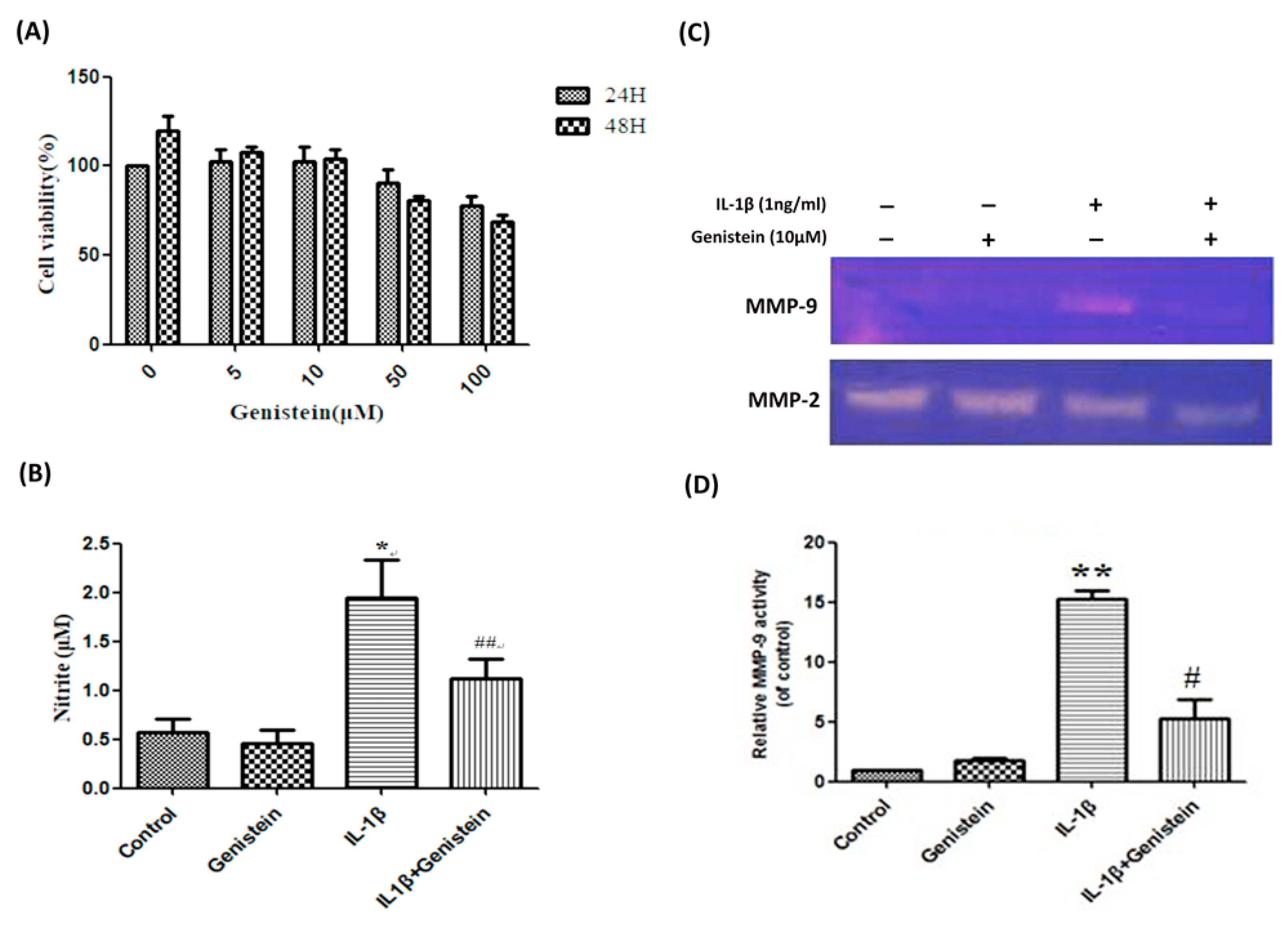
2.3. Cell Viability Assay
2.4. Measurement of Nitric Oxide (NO) Concentrations
2.5. Western Blotting
2.6. Gelatin Zymography
2.7. Nuclear Extract Preparation and Electrophoretic Mobility Shift Assay (EMSA)
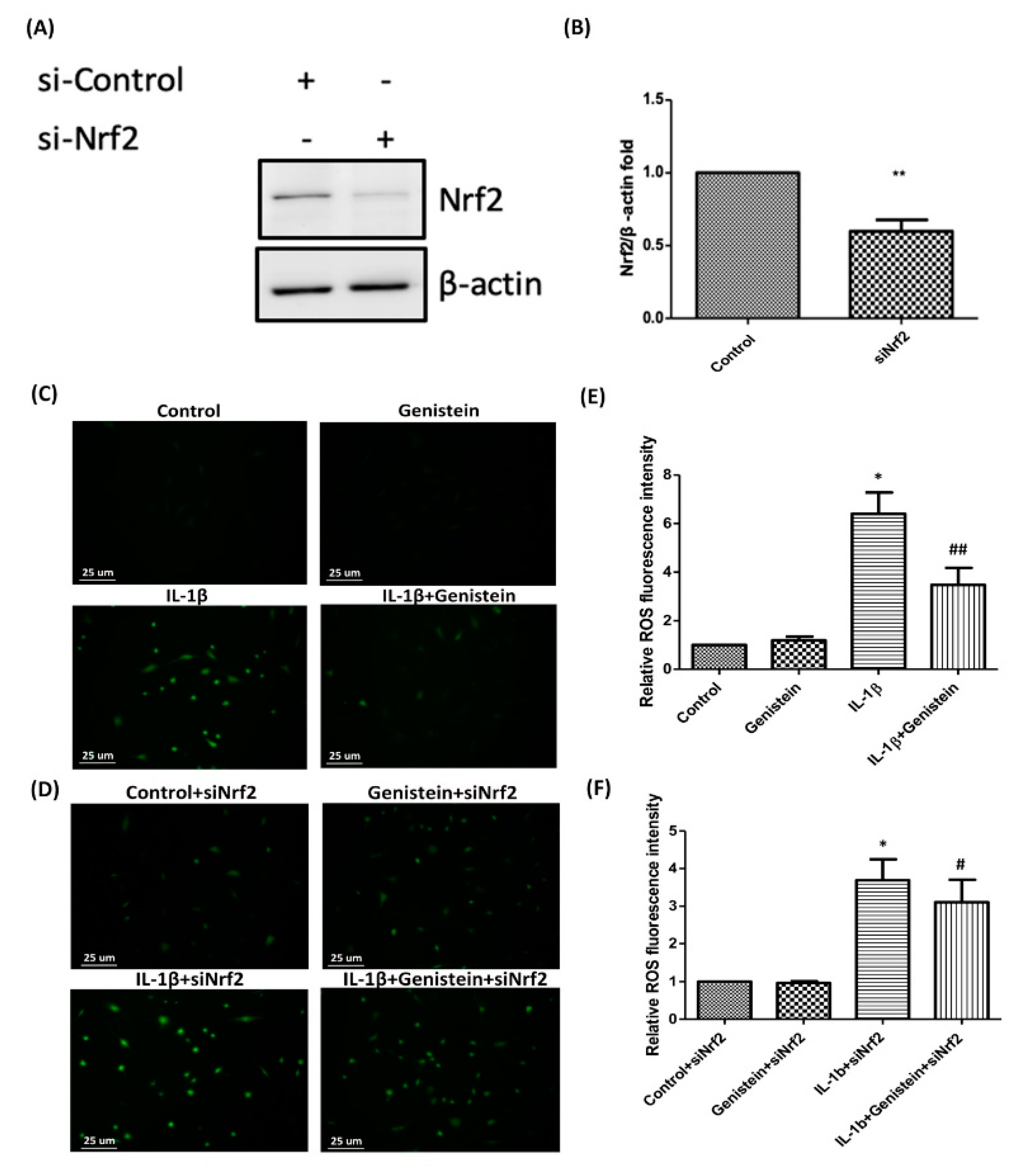
2.8. Transfection of Nrf2 siRNA and Measurement of Reactive Oxygen Species (ROS)
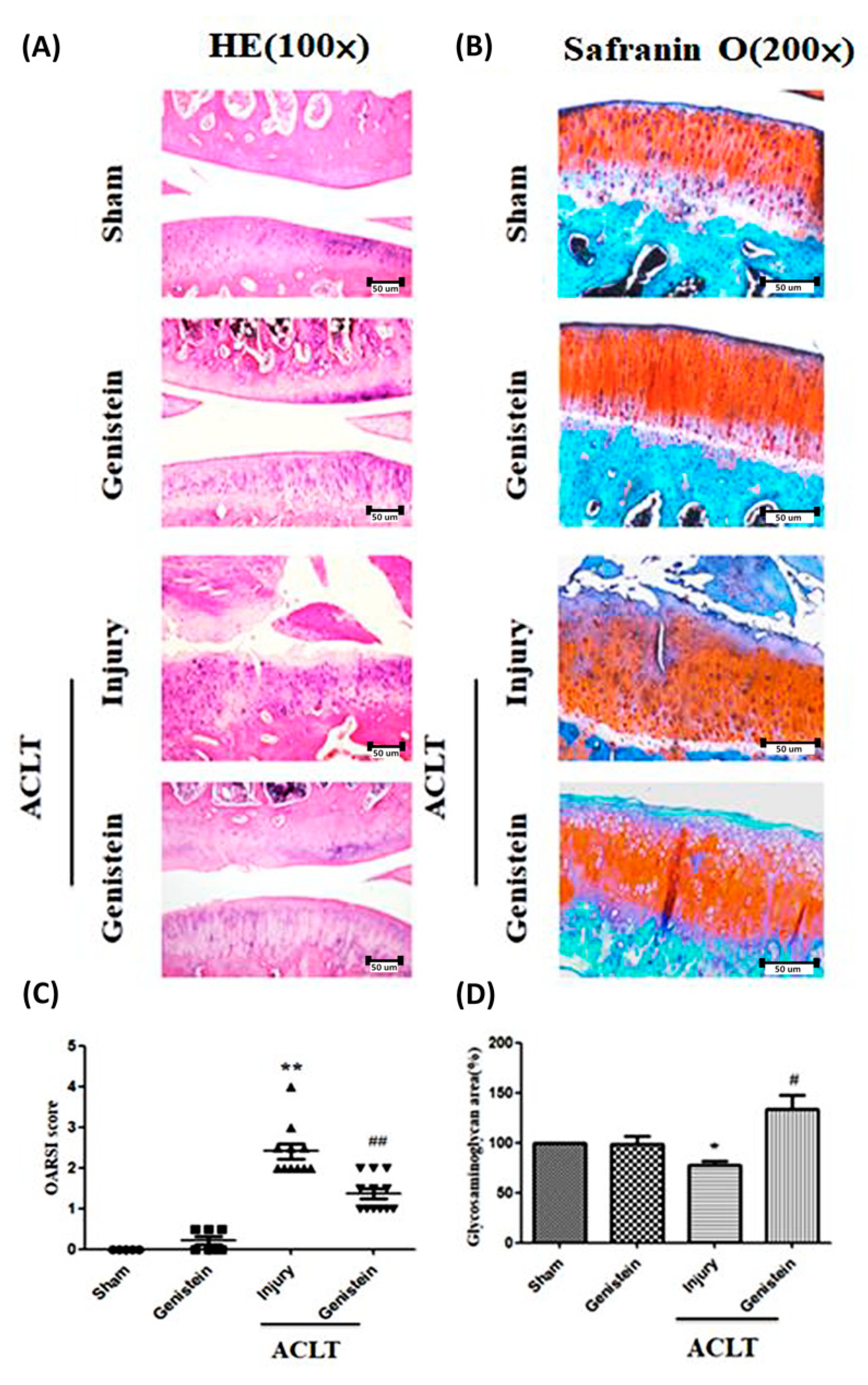
2.9. Anterior Cruciate Ligament Transection (ACLT) Rat Model
2.10. Hematoxylin and Eosin (H&E) Staining and Safranin-O Staining
2.11. Statistical Analysis
3. Results
3.1. Genistein Inhibited the Production of NO, Matrix Metalloproteinase-2 (MMP-2), and Matrix Metalloproteinase-9 (MMP-9) in Human OA Chondrocytes
3.2. Genistein Reduced Inflammation and Oxidative Stress in Human OA Chondrocytes
3.3. Effects of Genistein on the IL-1β-Induced Nrf2/HO-1 Pathway in Human OA Chondrocytes
3.4. Nrf2 siRNA Inhibits HO-1 Expression and Induces ROS Generation in IL-1β-Induced OA Chondrocytes
3.5. Genistein Slowed the Disease Progression of OA in an ACLT Rat Model
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal models of osteoarthritis: Challenges of model selection and analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000, 43, 1905–1915.
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Toghraie, F.S.; Chenari, N.; Gholipour, M.A.; Faghih, Z.; Torabinejad, S.; Dehghani, S.; Ghaderi, A. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 2011, 18, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthritis Cartilage 2015, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, M.; Coutts, R.D.; Amiel, D.; Hacker, S.A. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage 1996, 4, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Song, D.H.; Kim, S.H.; Jung, Y.; Kim, S.J. Development and characterization of various osteoarthritis models for tissue engineering. PLoS ONE 2018, 13, e0194288. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005, 44, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Takahashi, A.; de Andres, M.C.; Hashimoto, K.; Itoi, E.; Oreffo, R.O. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthritis Cartilage 2015, 23, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Santangelo, K.S.; Nuovo, G.J.; Bertone, A.L. In vivo reduction or blockade of interleukin-1beta in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthritis Cartilage 2012, 20, 1610–1618. [Google Scholar] [CrossRef]
- Wan, Z.H.; Zhao, Q. Gypenoside inhibits interleukin-1beta-induced inflammatory response in human osteoarthritis chondrocytes. J. Biochem. Mol. Toxicol 2017, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Chen, Y.; Zhou, B.; Shan, X.; Yang, G. Eriodictyol inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes. Biomed. Pharmacother 2018, 107, 1128–1134. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Buelna-Chontal, M.; Zazueta, C. Redox activation of Nrf2 & NF-kappaB: A double end sword? Cell Signal. 2013, 25, 2548–2557. [Google Scholar] [PubMed]
- Sun, C.C.; Li, S.J.; Yang, C.L.; Xue, R.L.; Xi, Y.Y.; Wang, L.; Zhao, Q.L.; Li, D.J. Sulforaphane Attenuates Muscle Inflammation in Dystrophin-deficient mdx Mice via NF-E2-related Factor 2 (Nrf2)-mediated Inhibition of NF-kappaB Signaling Pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar] [CrossRef]
- Kong, P.; Chen, G.; Jiang, A.; Wang, Y.; Song, C.; Zhuang, J.; Xi, C.; Wang, G.; Ji, Y.; Yan, J. Sesamin inhibits IL-1beta-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget 2016, 7, 83720–83726. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yin, S.; Yang, J.; Jiang, Q.; Cao, W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res. Ther. 2015, 17, 269. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Ding, X.; Xuan, J.; Hu, Z.; Wu, D.; Zhu, X.; Feng, Z.; Ni, W.; Wu, A. The protective effect of sinapic acid in osteoarthritis: In vitro and in vivo studies. J. Cell Mol. Med. 2019, 23, 1940–1950. [Google Scholar] [CrossRef] [Green Version]
- Egerbacher, M.; Helmreich, M.; Rossmanith, W.; Haeusler, G. Estrogen receptor-alpha and estrogen receptor-beta are present in the human growth plate in childhood and adolescence, in identical distribution. Horm Res. 2002, 58, 99–103. [Google Scholar] [CrossRef]
- Dayani, N.; Corvol, M.T.; Robel, P.; Eychenne, B.; Moncharmont, B.; Tsagris, L.; Rappaport, R. Estrogen receptors in cultured rabbit articular chondrocytes: Influence of age. J. Steroid. Biochem. 1988, 31, 351–356. [Google Scholar] [CrossRef]
- Ushiyama, T.; Ueyama, H.; Inoue, K.; Ohkubo, I.; Hukuda, S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage 1999, 7, 560–566. [Google Scholar] [CrossRef]
- Hooshmand, S.; Soung do, Y.; Lucas, E.A.; Madihally, S.V.; Levenson, C.W.; Arjmandi, B.H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem. 2007, 18, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Bellino, F.L. Estrogen metabolism, not biosynthesis, in rabbit articular cartilage and isolated chondrocytes: A preliminary study. Steroids 1992, 57, 507–510. [Google Scholar] [CrossRef]
- Cheng, P.; Ma, X.; Xue, Y.; Li, S.; Zhang, Z. Effects of estradiol on proliferation and metabolism of rabbit mandibular condylar cartilage cells in vitro. Chin. Med. J. (Engl.) 2003, 116, 1413–1417. [Google Scholar]
- Rosner, I.A.; Goldberg, V.M.; Moskowitz, R.W. Estrogens and osteoarthritis. Clin. Orthop. Relat. Res. 1986, 77–83. [Google Scholar] [CrossRef]
- Turner, A.S.; Athanasiou, K.A.; Zhu, C.F.; Alvis, M.R.; Bryant, H.U. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthritis Cartilage 1997, 5, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Liu, T.K.; Chen, T.J. Estrogen and osteoarthritis: A study of synovial estradiol and estradiol receptor binding in human osteoarthritic knees. Biochem. Biophys. Res. Commun. 1992, 183, 1287–1291. [Google Scholar] [CrossRef]
- Bassleer, C.T.; Franchimont, P.P.; Henrotin, Y.E.; Franchimont, N.M.; Geenen, V.G.; Reginster, J.Y. Effects of ipriflavone and its metabolites on human articular chondrocytes cultivated in clusters. Osteoarthritis Cartilage 1996, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.C.; Lu, J.W.; Chien, C.Y.; Huang, H.S.; Lee, C.C.; Lien, S.B.; Lin, L.C.; Chen, L.W.; Ho, Y.J.; Shen, M.C.; et al. Arthroprotective Effects of Cf-02 Sharing Structural Similarity with Quercetin. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Ho, L.J.; Lin, L.C.; Hung, L.F.; Wang, S.J.; Lee, C.H.; Chang, D.M.; Lai, J.H.; Tai, T.Y. Retinoic acid blocks pro-inflammatory cytokine-induced matrix metalloproteinase production by down-regulating JNK-AP-1 signaling in human chondrocytes. Biochem. Pharmacol. 2005, 70, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio. 2015, 5, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Piskin, A.; Gulbahar, M.Y.; Tomak, Y.; Gulman, B.; Hokelek, M.; Kerimoglu, S.; Koksal, B.; Alic, T.; Kabak, Y.B. Osteoarthritis models after anterior cruciate ligament resection and medial meniscectomy in rats. A histological and immunohistochemical study. Saudi. Med. J. 2007, 28, 1796–1802. [Google Scholar] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin Inhibits IL-1beta-Induced Production of Matrix Metalloproteinase in Articular Chondrocytes. Mol. Ther. Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Horwitz, K.B.; Ryan, D.S.; McGuire, W.L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology 1978, 103, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.L.; Torroella-Kouri, M.; Iragavarapu-Charyulu, V. Molecular events involved in the increased expression of matrix metalloproteinase-9 by T lymphocytes of mammary tumor-bearing mice. Int. J. Mol. Med. 2008, 21, 125–134. [Google Scholar] [CrossRef]
- Craft, C.S.; Xu, L.; Romero, D.; Vary, C.P.; Bergan, R.C. Genistein induces phenotypic reversion of endoglin deficiency in human prostate cancer cells. Mol. Pharmacol. 2008, 73, 235–242. [Google Scholar] [CrossRef]
- Somjen, D.; Amir-Zaltsman, Y.; Gayer, B.; Kulik, T.; Knoll, E.; Stern, N.; Lu, L.J.; Toldo, L.; Kohen, F. 6-Carboxymethyl genistein: A novel selective oestrogen receptor modulator (SERM) with unique, differential effects on the vasculature, bone and uterus. J. Endocrinol. 2002, 173, 415–427. [Google Scholar] [CrossRef]
- McMurray, R.W. Estrogen, prolactin, and autoimmunity: Actions and interactions. Int. Immunopharmacol 2001, 1, 995–1008. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Smith, B.J.; Sinichi, N.; Hodges, S.B.; Juma, S.; Munson, M.E.; Payton, M.E.; Tivis, R.D.; et al. Soy protein may alleviate osteoarthritis symptoms. Phytomedicine 2004, 11, 567–575. [Google Scholar] [CrossRef]
- Sheu, F.; Lai, H.H.; Yen, G.C. Suppression effect of soy isoflavones on nitric oxide production in RAW 264.7 macrophages. J. Agric. Food Chem. 2001, 49, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Laughton, M.J.; Evans, P.J.; Moroney, M.A.; Hoult, J.R.; Halliwell, B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem. Pharmacol. 1991, 42, 1673–1681. [Google Scholar] [CrossRef]
- Largo, R.; Alvarez-Soria, M.A.; Diez-Ortego, I.; Calvo, E.; Sanchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Ionescu, M.; Fitzcharles, M.A.; Billinghurst, R.C. The assessment of cartilage degradation in vivo: Development of an immunoassay for the measurement in body fluids of type II collagen cleaved by collagenases. J. Immunol. Methods 2004, 294, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Billinghurst, R.C.; Dahlberg, L.; Ionescu, M.; Reiner, A.; Bourne, R.; Rorabeck, C.; Mitchell, P.; Hambor, J.; Diekmann, O.; Tschesche, H.; et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 1997, 99, 1534–1545. [Google Scholar] [CrossRef]
- Borden, P.; Solymar, D.; Sucharczuk, A.; Lindman, B.; Cannon, P.; Heller, R.A. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J. Biol. Chem. 1996, 271, 23577–23581. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000, 43, 1916–1926. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. Genistein Protects Against Ox-LDL-Induced Inflammation Through MicroRNA-155/SOCS1-Mediated Repression of NF-kB Signaling Pathway in HUVECs. Inflammation 2017, 40, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Jang, H.D. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 2014, 15, 10605–10621. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Q.; Jin, S.; He, D.; Zhao, S.; Liu, S. Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 2008, 59, 405–412. [Google Scholar] [CrossRef]
- Mohammad-Shahi, M.; Haidari, F.; Rashidi, B.; Saei, A.A.; Mahboob, S.; Rashidi, M.R. Comparison of the effects of genistein and daidzein with dexamethasone and soy protein on rheumatoid arthritis in rats. Bioimpacts 2011, 1, 161–170. [Google Scholar]
- Hu, Y.; Li, J.; Qin, L.; Cheng, W.; Lai, Y.; Yue, Y.; Ren, P.; Pan, X.; Zhang, P. Study in Treatment of Collagen-Induced Arthritis in DBA/1 Mice Model by Genistein. Curr. Pharm. Des. 2016, 22, 6975–6981. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.B.; Xing, X.H.; Dong, G.Y.; Weng, X.L.; Wang, M.Q. Excess genistein suppresses the synthesis of extracellular matrix in female rat mandibular condylar cartilage. Acta Pharmacol. Sin. 2012, 33, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-C.; Wang, C.-C.; Lu, J.-W.; Lee, C.-H.; Chen, S.-C.; Ho, Y.-J.; Peng, Y.-J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051180
Liu F-C, Wang C-C, Lu J-W, Lee C-H, Chen S-C, Ho Y-J, Peng Y-J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients. 2019; 11(5):1180. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051180
Chicago/Turabian StyleLiu, Feng-Cheng, Chih-Chien Wang, Jeng-Wei Lu, Chian-Her Lee, Shao-Chi Chen, Yi-Jung Ho, and Yi-Jen Peng. 2019. "Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation" Nutrients 11, no. 5: 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051180




