Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Preparation of Placebo Yogurt and Yogurt Containing B. Animalis Subsp. Lactis and Arg (Bifal + Arg YG)
2.3. Experimental Design
2.4. EndoPAT
2.5. Meal Preparation and Fecal Collection Methods
2.6. Extraction of Fecal Bacterial DNA
2.7. Construction of the 16S rRNA Gene Amplicon Library and Next-Generation Sequencing
2.8. Data Processing and Sequence Alignment
2.9. Real-Time PCR for the Quantitative Determination of B. animalis subsp. lactis Cell Numbers
2.10. Determination of Fecal Polyamine Concentration
2.11. Determination of Serum Polyamine Concentration
2.12. Determination of Fecal Trimethylamine
2.13. Physical Examination, Urinalysis, and Blood Biochemical Analyses
2.14. Measurement of Serum NO2/NO3, Tumor Necrosis Factor (TNF)-α, and Interleukin (IL)-1β
2.15. Evaluation of Safety
2.16. Statistical Analysis
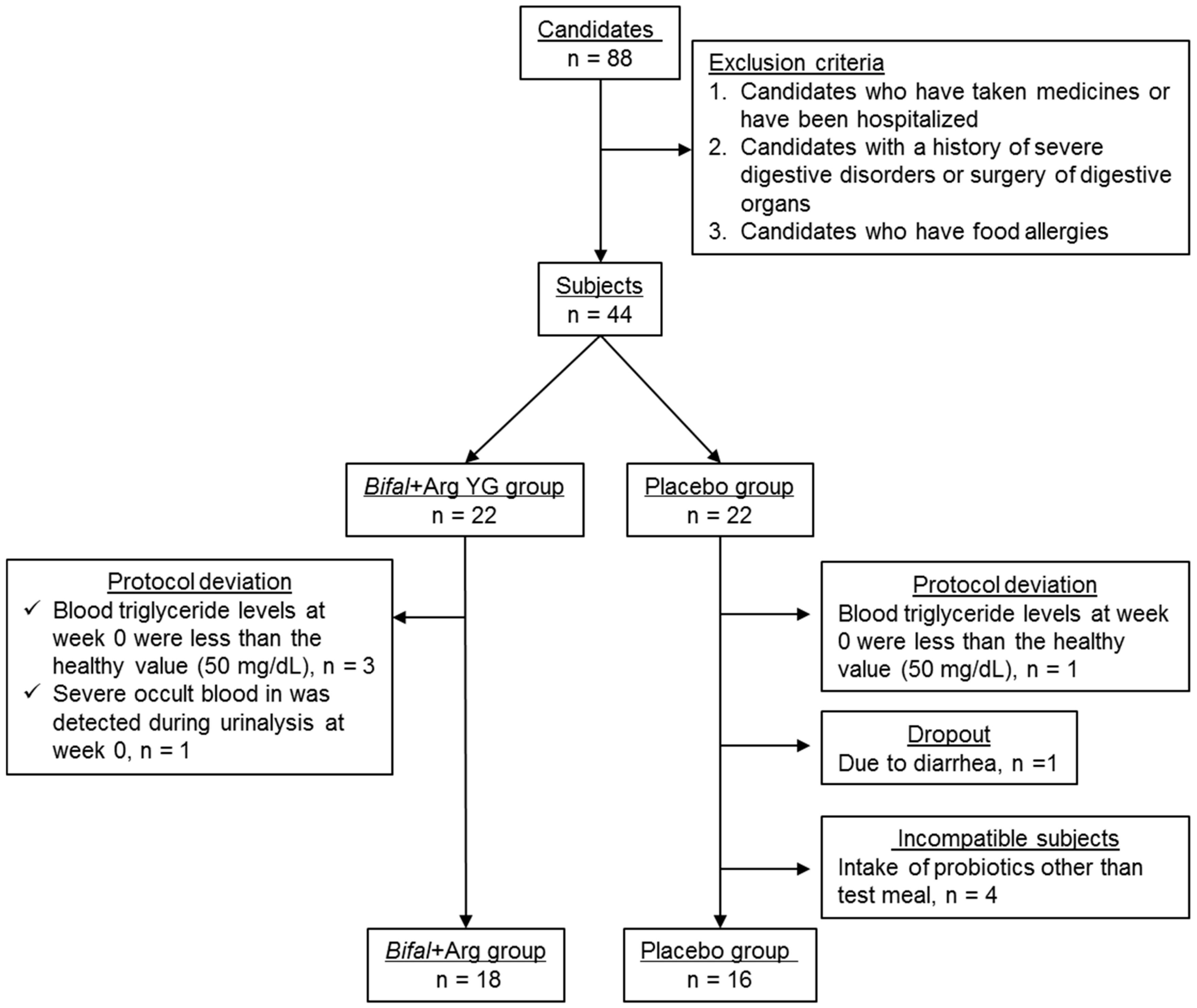
3. Results
3.1. Subjects
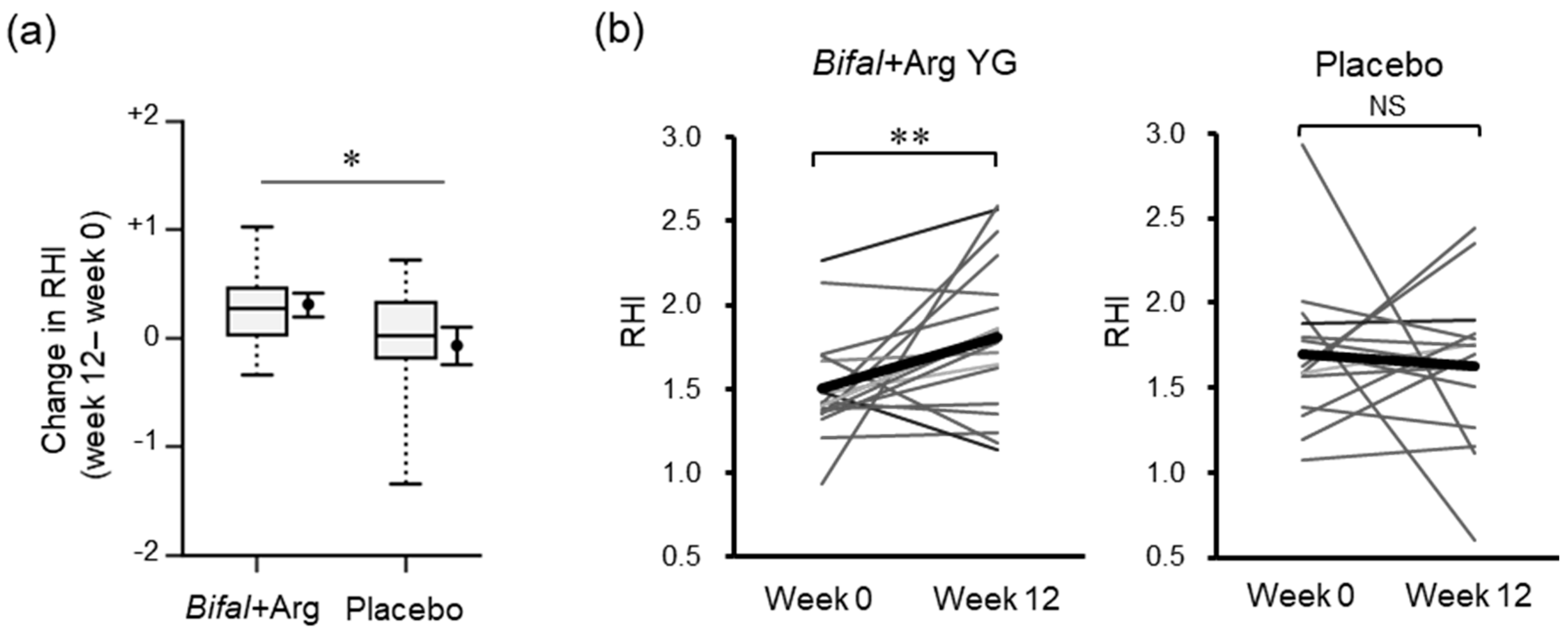
3.2. RHI
3.3. Systolic Blood Pressure and Diastolic Blood Pressure
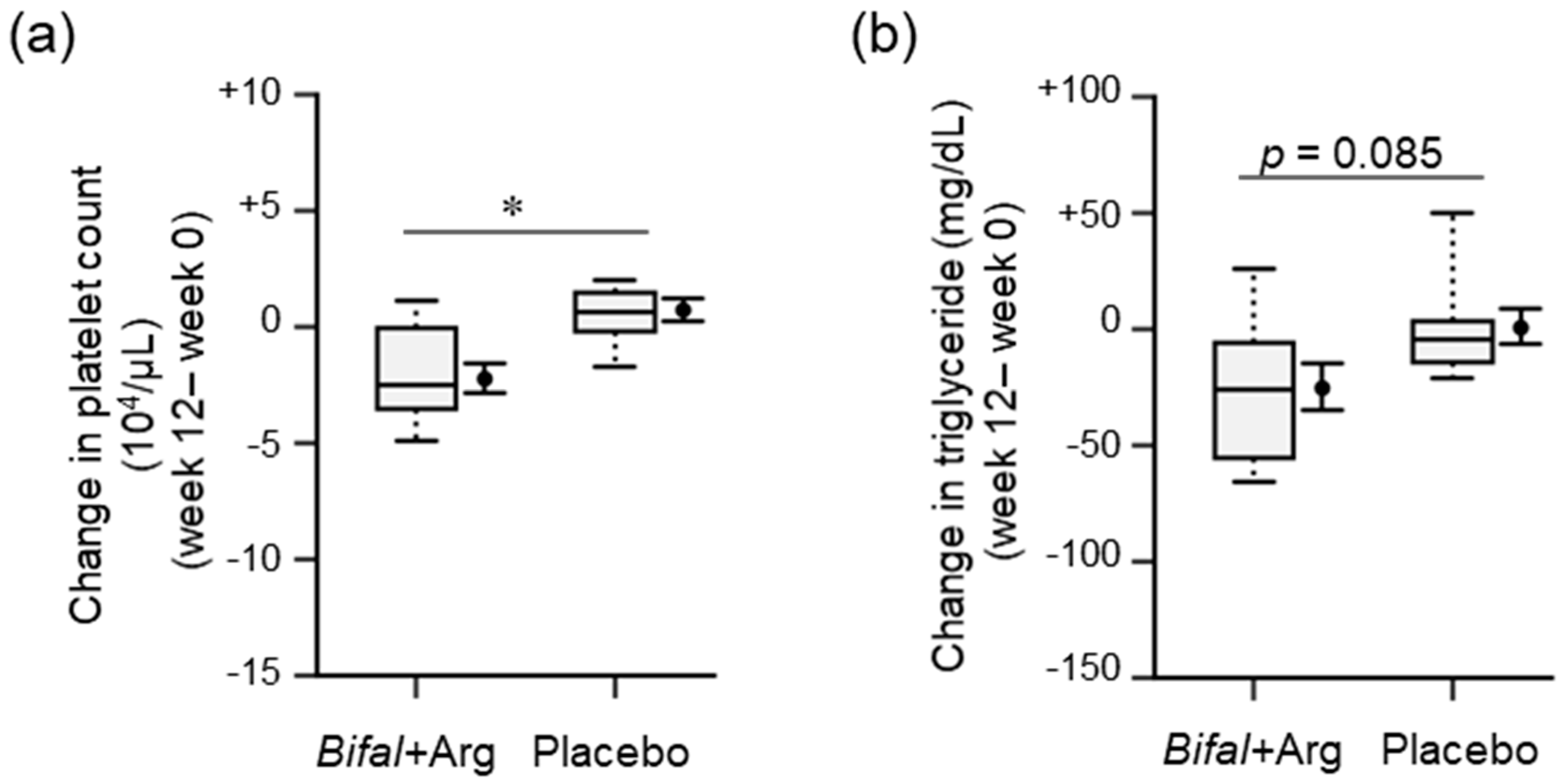
3.4. Physical Parameters, Urinalysis, and Blood Biochemical Analyses
3.5. Serum NO2/NO3, TNF-α, IL-1β, and Fecal Trimethylamine Concentrations
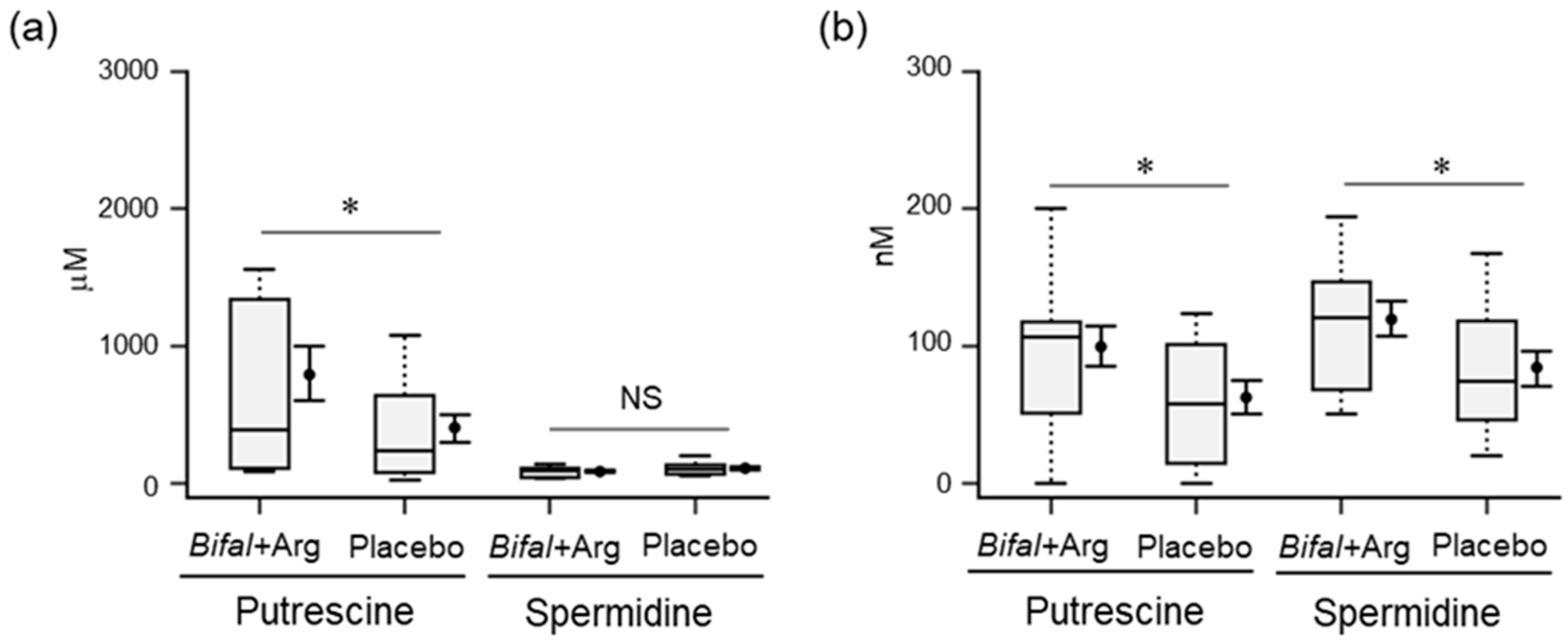
3.6. Polyamine Concentration in Feces and Serum
3.7. Fecal Microbiota
3.8. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [PubMed]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kanungo, M.S. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 1982, 17, 95–103. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Nakamura, T.; Kasono, K.; Kawakami, M.; Konishi, F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J. Immunol. 2005, 175, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease—An epidemiological study. Glob. J. Health Sci. 2012, 4, 170–178. [Google Scholar] [CrossRef]
- Soda, K. Polyamine intake, dietary pattern, and cardiovascular disease. Med. Hypotheses 2010, 75, 299–301. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [Green Version]
- Uda, K.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Rapid absorption of luminal polyamines in a rat small intestine ex vivo model. J. Gastroenterol. Hepatol. 2003, 18, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef]
- Gerner, E.W.; Meyskens, F.L., Jr. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Kitada, Y.; Muramatsu, K.; Toju, H.; Kibe, R.; Benno, Y.; Kurihara, S.; Matsumoto, M. Bioactive polyamine production by a novel hybrid system comprising multiple indigenous gut bacterial strategies. Sci. Adv. 2018, 4, eaat0062. [Google Scholar] [CrossRef] [Green Version]
- Dangardt, F.; Osika, W.; Chen, Y.; Nilsson, U.; Gan, L.M.; Gronowitz, E.; Strandvik, B.; Friberg, P. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis 2010, 212, 580–585. [Google Scholar] [CrossRef]
- Welfare, J.M.O. Guideline of Hyperlipidemia Treatment; Japan Medical Association: Tokyo, Japan, 1991. [Google Scholar]
- Committee, J.C.F.C.L.S.U.T.S. Japanese journal of clinical laboratory standards. 2004, 19, 53–65. [Google Scholar]
- Bruno, R.M.; Gori, T.; Ghiadoni, L. Endothelial function testing and cardiovascular disease: focus on peripheral arterial tonometry. Vasc. Health Risk Manag. 2014, 10, 577–584. [Google Scholar] [Green Version]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [Green Version]
- Yeo, T.W.; Lampah, D.A.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Mcneil, Y.R.; Darcy, C.J.; Granger, D.L.; Weinberg, J.B.; Lopansri, B.K.; et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J. Exp. Med. 2007, 204, 2693–2704. [Google Scholar] [CrossRef]
- Ohno, Y.; Hashiguchi, T.; Maenosono, R.; Yamashita, H.; Taira, Y.; Minowa, K.; Yamashita, Y.; Kato, Y.; Kawahara, K.; Maruyama, I. The diagnostic value of endothelial function as a potential sensor of fatigue in health. Vasc. Health Risk Manag. 2010, 6, 135–144. [Google Scholar] [Green Version]
- Syvanen, K.; Korhonen, P.; Partanen, A.; Aarnio, P. Endothelial function in a cardiovascular risk population with borderline ankle-brachial index. Vasc. Health Risk Manag. 2011, 7, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minister of Health Labour and Welfare (Japan). Dietary Reference Intakes for Japanese (2015). Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Full_DRIs2015.pdf (accessed on 19 May 2019).
- Matsumoto, M.; Kunisawa, A.; Hattori, T.; Kawana, S.; Kitada, Y.; Tamada, H.; Kawano, S.; Hayakawa, Y.; Iida, J.; Fukusaki, E. Free D-amino acids produced by commensal bacteria in the colonic lumen. Sci. Rep. 2018, 8, 17915. [Google Scholar] [CrossRef]
- Kim, S.W.; Suda, W.; Kim, S.; Oshima, K.; Fukuda, S.; Ohno, H.; Morita, H.; Hattori, M. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res. 2013, 20, 241–253. [Google Scholar] [CrossRef] [PubMed]
- QIIME. Available online: http://qiime.org/ (accessed on 20 March 2018).
- RDP Release 11, Update 5. Available online: https://rdp.cme.msu.edu/ (accessed on 20 March 2018).
- DDBJ Sequence Read Archive. Available online: http://trace.ddbj.nig.ac.jp/dra/ (accessed on 9 May 2018).
- Matsumoto, M.; Sakamoto, M.; Benno, Y. Dynamics of fecal microbiota in hospitalized elderly fed probiotic LKM512 yogurt. Microbiol. Immunol. 2009, 53, 421–432. [Google Scholar] [CrossRef]
- Chen, G.G.; Turecki, G.; Mamer, O.A. A quantitative GC-MS method for three major polyamines in postmortem brain cortex. J. Mass Spectrom. 2009, 44, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kitada, Y.; Shimomura, Y.; Naito, Y. Bifidobacterium animalis subsp. lactis LKM512 reduces levels of intestinal trimethylamine produced by intestinal microbiota in healthy volunteers: A double-blind, placebo-controlled study. J. Funct. Foods 2017, 36, 94–101. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Abi-Hanna, A.; Moore, N.; Yolken, R.H. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am. J. Clin. Nutr. 2004, 79, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M.; Tynkkynen, S.; Mattila-Sandholm, T.; De Vos, W.M. Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Biotechnol. 2005, 16, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Brands, M.W.; Daniels, S.R.; Karanja, N.; Elmer, P.J.; Sacks, F.M.; American Heart, A. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006, 47, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Milovic, V.; Faust, D.; Turchanowa, L.; Stein, J.; Caspary, W.F. Permeability characteristics of polyamines across intestinal epithelium using the Caco-2 monolayer system: comparison between transepithelial flux and mitogen-stimulated uptake into epithelial cells. Nutrition 2001, 17, 462–466. [Google Scholar] [CrossRef]
- Bardocz, S.; Duguid, T.J.; Brown, D.S.; Grant, G.; Pusztai, A.; White, A.; Ralph, A. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 1995, 73, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moinard, C.; Cynober, L.; De Bandt, J.P. Polyamines: metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.B.; Kim, M.M. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int. J. Biol. Macromol. 2014, 63, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Larocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M., Jr.; Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 2013, 134, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Thaulow, E.; Erikssen, J.; Sandvik, L.; Stormorken, H.; Cohn, P.F. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation 1991, 84, 613–617. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Boger, R.H.; Galland, A.; Tsikas, D.; Frolich, J.C. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Kablak-Ziembicka, A.; Przewlocki, T.; Sokolowski, A.; Tracz, W.; Podolec, P. Carotid intima-media thickness, hs-CRP and TNF-alpha are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis 2011, 214, 185–190. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Winkler, T.; Heinsen, F.A.; Ruhlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017, 4, 282–290. [Google Scholar] [CrossRef]
- Kamo, T.; Akazawa, H.; Suda, W.; Saga-Kamo, A.; Shimizu, Y.; Yagi, H.; Liu, Q.; Nomura, S.; Naito, A.T.; Takeda, N.; et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PloS one 2017, 12, e0174099. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Troseid, M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J. Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2015, 94, e1714. [Google Scholar] [CrossRef] [PubMed]
- Tripolt, N.J.; Leber, B.; Blattl, D.; Eder, M.; Wonisch, W.; Scharnagl, H.; Stojakovic, T.; Obermayer-Pietsch, B.; Wascher, T.C.; Pieber, T.R.; et al. Short communication: Effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, beta-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome—A pilot study. J. Dairy Sci. 2013, 96, 89–95. [Google Scholar] [CrossRef] [PubMed]

| Bifal + Arg Mean (SEM) | Placebo Mean (SEM) | |||||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Change | Week 0 | Week 12 | Change | |
| Height, cm | 163.7 (2.1) | 163.9 (2.0) | +0.1 (0.1) | 162.3 (2.2) | 162.3 (2.2) | +0.1 (0.2) |
| Body weight, kg | 64.0 (2.5) | 64.5 (2.5) | +0.5 (0.3) | 63.7 (2.6) | 64.6 (2.6) | +0.9 (0.6) |
| Abdominal circumference, cm | 79.4 (2.3) | 82.8 (2.3) †† | +3.4 (0.6) | 80.2 (2.2) | 84.3 (1.8) †† | +4.1 (0.9) |
| Heart rate, bpm | 72.9 (1.7) | 70.9 (1.9) | −2.0 (2.0) # | 69.7 (1.5) | 73.2 (2.4) | +3.5 (2.5) # |
| BMI | 23.8 (0.7) | 23.9 (0.7) | +0.2 (0.1) | 24.1 (0.6) | 24.4 (0.7) | +0.3 (0.2) |
| RHI | 1.50 (0.07) | 1.81 (0.11) † | +0.31 (0.12) # | 1.68 (0.11) | 1.63 (0.12) | −0.07(0.18) # |
| Systolic blood pressure, mmHg | 122.4 (2.3) | 120.2 (2.8) | −2.2 (2.3) | 123.1 (3.4) | 125.3 (2.5) | +2.1 (2.7) |
| Diastolic blood pressure, mmHg | 77.9 (1.5) | 75.1 (2.2) | −2.9 (2.1) | 81.2 (2.2) | 78.9 (1.9) | −2.25 (1.5) |
| Bifal + Arg Mean (SEM) | Placebo Mean (SEM) | |||||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Change | Week 0 | Week 12 | Change | |
| White blood cell count, μL | 6315 (316) | 5945 (326) | −370 (307) | 5641 (347) | 5352 (240) | −123 (395) |
| Erythrocyte count, 104/μL | 482.7(11.7) | 478.6 (10.9) | −4.1 (5.1) | 479.6(12.1) | 470.5 (11.5) † | −9.1 (3.2) |
| Hemoglobin, g/dL | 14.0 (0.44) | 14.0 (0.4) | −0.01 (0.24) | 13.9 (0.4) | 13.6 (0.38) † | −0.32 (0.12) |
| Hematocrit, % | 43.5 (1.1) | 43.4 (1.1) | −0.04 (0.5) | 42.8 (0.9) | 42.4 (0.98) | −0.44 (0.34) |
| Platelet count, 104/μL | 27.9 (1.4) | 25.6 (1.1) *,†† | −2.2 (0.6) # | 27.7 (1.0) | 28.4 (1.0) * | +0.7 (0.5) # |
| Blood glucose level. mg/dL | 89.17(2.92) | 87.6 (2.2) | −1.56 (2.24) | 92.31 (3.5) | 91.13 (3.54) | +0.07 (2.59) |
| Total cholesterol, mg/dL | 197.2 (6.6) * | 196.3(6.0) ** | −0.9 (4.4 | 223.8 (9.1) * | 223.1(7.8) ** | −0.7 (5.3) |
| Triglyceride, mg/dL | 129.3(15.0) | 103.9(12.9) † | −25.4 (10.5) | 104.8 (15.7) | 125.1 (22.3) | +0.6 (8.0) |
| HDL-cholesterol, mg/dL | 57.4 (3.3) | 60.4 (3.4) † | +3.0 (1.2) | 54.4 (3.1) | 55.6 (3.1) | +1.21 (1.8) |
| LDL-cholesterol, mg/dL | 121.7 (6.9) | 119.7 (6.3) | −2.0 (3.5) | 129.2 (10.5) | 131.1 (9.3) | +1.9 (4.3) |
| NO2/NO3, mM | N.T. | 21.3 (1.5) | N.T. | N.T. | 24.5 (2.1) | N.T |
| TNF-a, mM | N.T. | 0.552 (0.03) | N.T. | N.T. | 0.617 (0.05) | N.T. |
| IL-1, mM | N.T. | 0.046 (0.01) | N.T. | N.T. | 0.039 (0.01) | N.T. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1188. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051188
Matsumoto M, Kitada Y, Naito Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients. 2019; 11(5):1188. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051188
Chicago/Turabian StyleMatsumoto, Mitsuharu, Yusuke Kitada, and Yuji Naito. 2019. "Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial" Nutrients 11, no. 5: 1188. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051188





