Advanced Glycation End-Products Can Activate or Block Bitter Taste Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Molecular Biology and Cell Culture
2.3. Functional Assays
2.4. Molecular Modeling and Ligand Docking
2.5. Statistical Analysis
3. Results
3.1. Prediction of Binding Affinities of AGEs for T2R4
3.2. GOLD and CML Inhibit Activation of T2R4
3.3. Analysis of the Binding Pocket for AGEs in T2R4
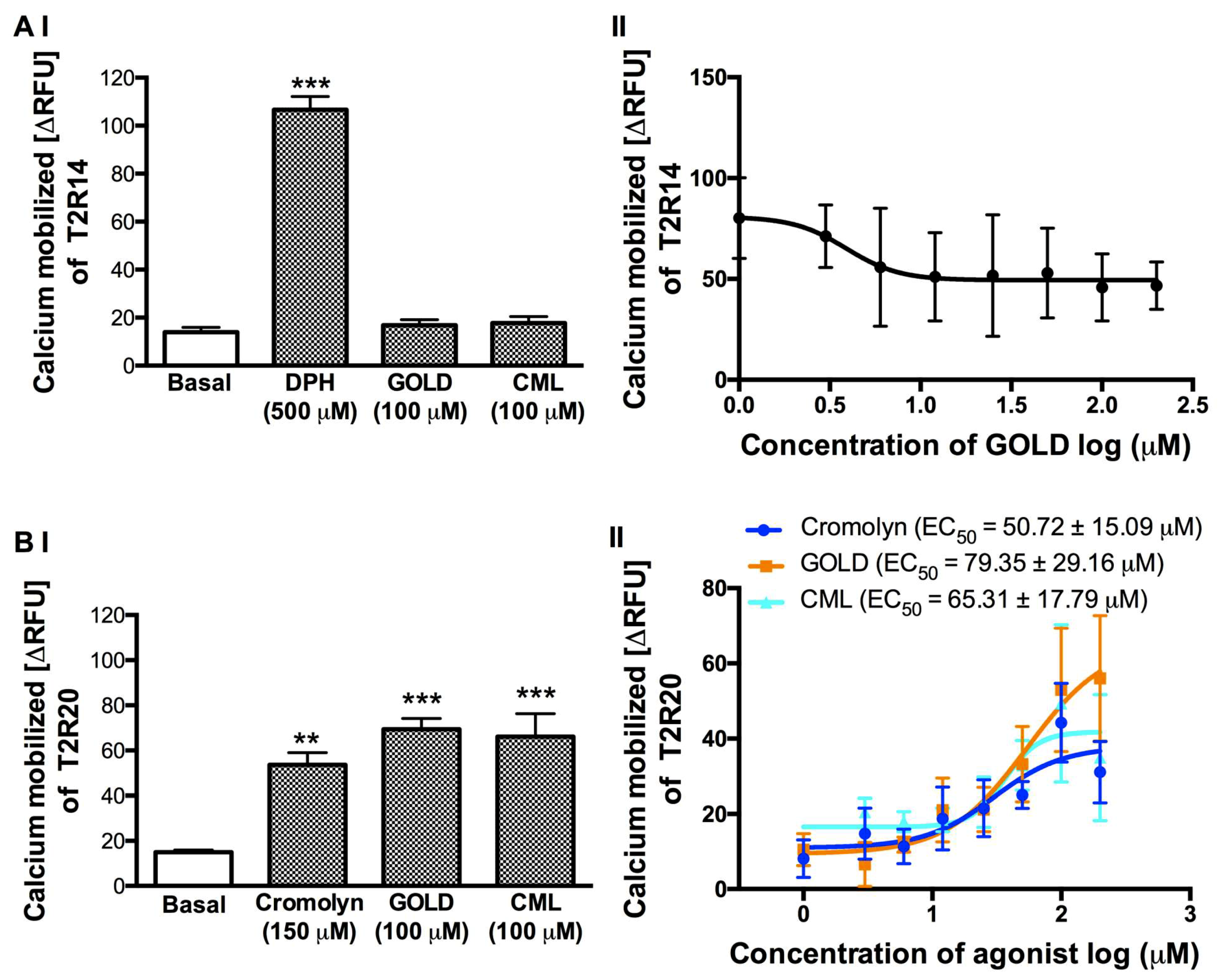
3.4. Effect of GOLD and CML on the Activation of T2R14 and T2R20
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGE | advanced glycation end-product |
| BCML | Nα,Nα-bis(carboxymethyl)-l-lysine |
| CML | Nε-carboxymethyl-l-lysine |
| DPH | diphenhydramine |
| GOLD | glyoxal-derived lysine dimer |
| HEK293T | human embryonic kidney cells |
| RAGE | receptor for advanced glycation end-products |
| RFU | relative fluorescence units |
| T2Rs | bitter taste receptors |
References
- Wu, S.V.; Rozengurt, N.; Yang, M.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Expression of bitter taste receptors of the t2r family in the gastrointestinal tract and enteroendocrine stc-1 cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem. Senses 2012, 37, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef]
- Shaik, F.A.; Singh, N.; Arakawa, M.; Duan, K.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Extraoral roles in pathophysiology. Int. J. Biochem. Cell Biol. 2016, 77, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M.S.; Dubovski, N.; Margulis, E.; Niv, M.Y. Bitterdb: Taste ligands and receptors database in 2019. Nucleic. Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Howard, R.; Upadhyaya, J.D.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Novel insights into the biochemistry and pharmacology. Int. J. Biochem. Cell Biol. 2016, 77, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.P.; Brockhoff, A.; Batram, C.; Menzel, S.; Sonnabend, C.; Born, S.; Galindo, M.M.; Kohl, S.; Thalmann, S.; Ostopovici-Halip, L.; et al. Modulation of bitter taste perception by a small molecule htas2r antagonist. Curr. Biol. 2010, 20, 1104–1109. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Roudnitzky, N.; Appendino, G.; Avonto, C.; Meyerhof, W. Receptor agonism and antagonism of dietary bitter compounds. J. Neurosci. 2011, 31, 14775–14782. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human tas2r bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Felton, L.A. Use of polymers for taste-masking pediatric drug products. Drug Dev. Ind. Pharm. 2018, 44, 1049–1055. [Google Scholar] [CrossRef]
- Behrens, M.; Blank, K.; Meyerhof, W. Blends of non-caloric sweeteners saccharin and cyclamate show reduced off-taste due to tas2r bitter receptor inhibition. Cell Chem. Biol. 2017, 24, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Pydi, S.P.; Sobotkiewicz, T.; Billakanti, R.; Bhullar, R.P.; Loewen, M.C.; Chelikani, P. Amino acid derivatives as bitter taste receptor (t2r) blockers. J. Biol. Chem. 2014, 289, 25054–25066. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Li, L.; Deng, Y. Identification of age-precursors and age formation in glycation-induced bsa peptides. BMB Rep. 2008, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Brownlee, M.; Cerami, A. Accumulation of diabetic rat peripheral nerve myelin by macrophages increases with the presence of advanced glycosylation endproducts. J. Exp. Med. 1984, 160, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, L.; Li, B.; Zhao, D.; Li, Y.; Xu, Z.; Liu, G. Review of the characteristics of food-derived and endogenous ne-carboxymethyllysine. J. Food Prot. 2013, 76, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlender, J.M.; Franke, S.; Stein, G.; Wolf, G. Advanced glycation end products and the kidney. Am. J. Physiol. Renal. Physiol. 2005, 289, F645–F659. [Google Scholar] [CrossRef]
- Nicholl, I.D.; Bucala, R. Advanced glycation endproducts and cigarette smoking. Cell Mol. Biol. 1998, 44, 1025–1033. [Google Scholar]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef]
- Sjoberg, J.S.; Bulterijs, S. Characteristics, formation, and pathophysiology of glucosepane: A major protein cross-link. Rejuvenation Res. 2009, 12, 137–148. [Google Scholar] [CrossRef]
- Stitt, A.W.; Bucala, R.; Vlassara, H. Atherogenesis and advanced glycation: Promotion, progression, and prevention. Ann. N Y Acad. Sci. 1997, 811, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W.N. Epsilon.-(carboxymethyl) lysine is a dominant advanced glycation end product (age) antigen in tissue proteins. Biochemistry 1995, 34, 10872–10878. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Semba, R.D.; Sun, K.; Crasto, C.; Varadhan, R.; Bandinelli, S.; Fink, J.C.; Guralnik, J.M.; Ferrucci, L. Endogenous secretory receptor for advanced glycation end products and chronic kidney disease in the elderly population. Am. J. Nephrol. 2011, 33, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.A.; McCance, D.R.; Thorpe, S.R.; Lyons, T.J.; Baynes, J.W. Age-dependent accumulation of n epsilon-(carboxymethyl)lysine and n epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry 1991, 30, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Horvat, S.; Jakas, A. Peptide and amino acid glycation: New insights into the maillard reaction. J. Pept. Sci. 2004, 10, 119–137. [Google Scholar] [CrossRef]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Zheng, F.; Striker, G.E.; Vlassara, H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am. J. Pathol. 2008, 173, 327–336. [Google Scholar] [CrossRef]
- Zhang, C.; Alashi, A.M.; Singh, N.; Liu, K.; Chelikani, P.; Aluko, R.E. Beef protein-derived peptides as bitter taste receptor t2r4 blockers. J. Agric. Food Chem. 2018, 66, 4902–4912. [Google Scholar] [CrossRef]
- Xu, Q.; Singh, N.; Hong, H.; Yan, X.; Yu, W.; Jiang, X.; Chelikani, P.; Wu, J. Hen protein-derived peptides as the blockers of human bitter taste receptors t2r4, t2r7 and t2r14. Food Chem. 2019, 283, 621–627. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.A.; Wang, W.C.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Manson, M.L.; Safholm, J.; Al-Ameri, M.; Bergman, P.; Orre, A.C.; Sward, K.; James, A.; Dahlen, S.E.; Adner, M. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur. J. Pharmacol. 2014, 740, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.R.; Porrello, E.R.; Purdue, B.; Chan, H.W.; Voigt, A.; Frenzel, S.; Hannan, R.D.; Moritz, K.M.; Simmons, D.G.; Molenaar, P.; et al. Expression, regulation and putative nutrient-sensing function of taste gpcrs in the heart. PLoS ONE 2013, 8, e64579. [Google Scholar] [CrossRef] [PubMed]
- Orsmark-Pietras, C.; James, A.; Konradsen, J.R.; Nordlund, B.; Soderhall, C.; Pulkkinen, V.; Pedroletti, C.; Daham, K.; Kupczyk, M.; Dahlen, B.; et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur. Respir. J. 2013, 42, 65–78. [Google Scholar] [CrossRef]
- Foster, S.R.; Roura, E.; Thomas, W.G. Extrasensory perception: Odorant and taste receptors beyond the nose and mouth. Pharmacol. Ther. 2014, 142, 41–61. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Singh, N.; Upadhyaya, J.; Sikarwar, A.S.; Arakawa, M.; Dakshinamurti, S.; Bhullar, R.P.; Duan, K.; Chelikani, P. Analysis of the expression of human bitter taste receptors in extraoral tissues. Mol. Cell Biochem. 2017, 426, 137–147. [Google Scholar] [CrossRef]
- Levit, A.; Nowak, S.; Peters, M.; Wiener, A.; Meyerhof, W.; Behrens, M.; Niv, M.Y. The bitter pill: Clinical drugs that activate the human bitter taste receptor tas2r14. FASEB J. 2014, 28, 1181–1197. [Google Scholar] [CrossRef]
- Ji, M.; Su, X.; Su, X.; Chen, Y.; Huang, W.; Zhang, J.; Gao, Z.; Li, C.; Lu, X. Identification of novel compounds for human bitter taste receptors. Chem. Biol. Drug Des. 2014, 84, 63–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, X.; Peng, S.; Wang, S.; Huang, C.Z.; Huang, C.Z.; Zhang, Q.; Li, D.; Jiang, J.; et al. Identification of a specific agonist of human tas2r14 from radix bupleuri through virtual screening, functional evaluation and binding studies. Sci. Rep. 2017, 7, 12174. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Singh, N.; De Jesus, V.C.; Gounni, M.S.; Dhanaraj, P.; Chelikani, P. Chemosensory bitter taste receptors (t2rs) are activated by multiple antibiotics. FASEB J. 2019, 33, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Singh, N.; Jesus, V.C.; Duan, K.; Chelikani, P. Characterization of the binding sites for bacterial acyl homoserine lactones (ahls) on human bitter taste receptors (t2rs). ACS Infect. Dis. 2018, 4, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, J.D.; Chakraborty, R.; Shaik, F.A.; Jaggupilli, A.; Bhullar, R.P.; Chelikani, P. The pharmacochaperone activity of quinine on bitter taste receptors. PLoS ONE 2016, 11, e0156347. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Xu, B.; Bhullar, R.P.; Chelikani, P. Expression of g protein-coupled receptors in mammalian cells. Methods Enzymol. 2015, 556, 267–281. [Google Scholar] [PubMed]
- Liu, K.; Jaggupilli, A.; Premnath, D.; Chelikani, P. Plasticity of the ligand binding pocket in the bitter taste receptor t2r7. Biochim. Biophys. Acta. 2018, 5, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Dhanaraj, P.; Pritchard, A.; Sorensen, J.L.; Dakshinamurti, S.; Chelikani, P. Study of adenylyl cyclase-galphas interactions and identification of novel ac ligands. Mol. Cell Biochem. 2018, 446, 63–72. [Google Scholar] [CrossRef]
- Momany, F.A.; Rone, R. Validation of the general-purpose quanta(r)3.2/charmm(r) force-field. J. Comput. Chem. 1992, 13, 888–900. [Google Scholar] [CrossRef]
- Venkatapathy, R.; Wang, N.C. Developmental toxicity prediction. Methods Mol. Biol. 2013, 930, 305–340. [Google Scholar]
- Wu, G.; Robertson, D.H.; Brooks, C.L., 3rd; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of cdocker-a charmm-based md docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Kumar, M.; Pydi, S.P.; Sharma, S.; Singh, T.P.; Kaur, P. Identification of a high affinity selective inhibitor of polo-like kinase 1 for cancer chemotherapy by computational approach. J. Mol. Graph. Model. 2014, 51, 104–112. [Google Scholar] [CrossRef]
- Pymol: An Open-Source Molecular Graphics Tool. Available online: https://www.ccp4.ac.uk/newsletters/newsletter40/11_pymol.pdf (accessed on 20 May 2019).
- Soares, S.; Silva, M.S.; Garcia-Estevez, I.; Grobetamann, P.; Bras, N.; Brandao, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human bitter taste receptors are activated by different classes of polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R.; Nowak, S.; Di Pizio, A.; Kitaneh, H.; Abu-Jaish, A.; Meyerhof, W.; Niv, M.Y.; Behrens, M. Probing the binding pocket of the broadly tuned human bitter taste receptor tas2r14 by chemical modification of cognate agonists. Chem. Biol. Drug Des. 2016, 88, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.L.; Chang, C.I.; Chuang, K.P.; Wang, C.Y.; Liu, H.J. Advanced glycation end products down-regulate gap junctions in human hepatoma skhep 1 cells via the activation of src-dependent erk1/2 and jnk/sapk/ap1 signaling pathways. J. Agric. Food Chem. 2010, 58, 8636–8642. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Araya, P.; Romero, J.; Delgado-Lopez, F.; Gonzalez, I.; Anazco, C.; Perez-Castro, R. Skewed signaling through the receptor for advanced glycation end-products alters the proinflammatory profile of tumor-associated macrophages. Cancer Microenviron. 2018, 11, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. Rage and its ligands: Molecular interplay between glycation, inflammation, and hallmarks of cancer-a review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef] [PubMed]


| AGE Compound | Chemical Identifier | Predicted Binding Affinity, –log (Kd) | Predicted Kd (μM) |
|---|---|---|---|
| Pentosidine | CID 119593 | 7.71 | 0.02 |
| Nε-Carboxymethyl-lysine (CML) | CID 123800 | 7.34 | 0.04 |
| Tetrahydropyrimidine (THP) | CID 5231957 | 7.3 | 0.05 |
| 3-deoxyglucosone-derived lysine dimer (DOLD) | NA | 7.13 | 0.07 |
| Nε-Carboxyethyl Lysine (CEL) | CID 23400779 | 7.04 | 0.09 |
| Glyoxal-derived lysine dimer (GOLD) | CHEBI:59965 | 6.96 | 0.11 |
| Argpyrimidine | CID 17750123 | 6.81 | 0.15 |
| Methyl glyoxal hydroimidazolone 1(MG-H1) | NA | 6.77 | 0.17 |
| Methyl glyoxal-derived lysine dimer (MOLD) | NA | 6.6 | 0.25 |
| 3-deoxyglucosone hydroimidazolone 3 (3DG-H3) | NA | 6.51 | 0.31 |
| Fructosyl Lysine | CID 123708 | 6.47 | 0.34 |
| Pyrraline | CID 122228 | 6.22 | 0.60 |
| Nε-carboxymethyl-hydroxylysine (CMhL) | CID 125438 | 6.21 | 0.62 |
| Glucosepane | CSID 26333276 | 5.92 | 1.20 |
| 3-deoxyglucosone hydroimidazolone 2 (3DG-H2) | NA | 5.78 | 1.66 |
| ImidazoloneA | CSID 9993693 | 5.72 | 1.90 |
| 1-Alkyl-2-formyl-3,4-glycosyl-pyrrole (AFGP) | NA | 5.66 | 2.19 |
| Methyl glyoxal hydroimidazolone 2 (MG-H2) | NA | 5.62 | 2.40 |
| ImidazoloneB | CSID 9993693 | 5.29 | 5.13 |
| 3-deoxyglucosone hydroimidazolone 1 (3DG-H1) | NA | 4.77 | 16.9 |
| Glyoxal-derived hydroimidazolone (G-H) | NA | 4.7 | 19.9 |
| Methyl glyoxal hydroimidazolone 3 (MG-H3) | NA | 4.19 | 64.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaggupilli, A.; Howard, R.; Aluko, R.E.; Chelikani, P. Advanced Glycation End-Products Can Activate or Block Bitter Taste Receptors. Nutrients 2019, 11, 1317. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061317
Jaggupilli A, Howard R, Aluko RE, Chelikani P. Advanced Glycation End-Products Can Activate or Block Bitter Taste Receptors. Nutrients. 2019; 11(6):1317. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061317
Chicago/Turabian StyleJaggupilli, Appalaraju, Ryan Howard, Rotimi E. Aluko, and Prashen Chelikani. 2019. "Advanced Glycation End-Products Can Activate or Block Bitter Taste Receptors" Nutrients 11, no. 6: 1317. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061317





