Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds
Abstract
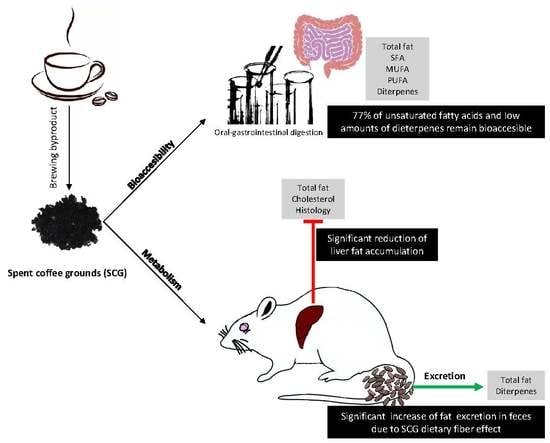
:1. Introduction
2. Materials and Methods
2.1. SCGs Samples
2.2. Safety of SCGs
2.2.1. Mycotoxins
2.2.2. Acute Toxicity Study
2.3. Fat, Fatty Acid Profile, and Diterpenes in SCGs
2.4. Bioaccesibility of Lipids and Diterpenes in SCGs
2.5. Pilot Repeated Dose Animal Study
2.5.1. Metabolism Study
Determination of Fat and Cholesterol in Rat Livers
Histopathological Examination
2.5.2. Lipid and Diterpenes Excretion
2.5.3. Dietary Fiber Effect Study (Gastrointestinal Motility)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Safety of SCGs
3.2. In Vitro Bioaccesibility of Lipids and Diterpens Composing SCGs
3.3. In Vivo Metabolism and Excretion of Lipids Composing SCGs
3.4. Gastrointestinal Motility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Padmapriya, R.; Tharian, J.; Thirunalasundari, T. Coffee waste management-An overview. Int. J. Curr. Sci. 2013, 9, 83–91. [Google Scholar]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Tamargo García, A.; Domínguez Pérez, I.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef] [PubMed]
- del Castillo, M.D.; Martinez-Saez, N.; Ullate, M. Healthy Bakery Products with High Level of Dietary Antioxidant Fibre. International Patent WO2014/128320Al, 2014. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Simões, J.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydr. Polym. 2013, 97, 81–89. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Calligaris, S.; Munari, M.; Arrighetti, G.; Barba, L. Insights into the physicochemical properties of coffee oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1270–1277. [Google Scholar] [CrossRef]
- George, S.E.; Ramalakshmi, K.; Rao, L.J.M. A perception on health benefits of coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef]
- Ricketts, M.-L.; Boekschoten, M.V.; Kreeft, A.J.; Hooiveld, G.J.E.J.; Moen, C.J.A.; Müller, M.; Frants, R.R.; Kasanmoentalib, S.; Post, S.M.; Princen, H.M.G.; et al. The Cholesterol-Raising Factor from Coffee Beans, Cafestol, as an Agonist Ligand for the Farnesoid and Pregnane X Receptors. Mol. Endocrinol. 2007, 21, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.M.; Vieira, G.S.; Hubinger, M.D. Influence of different combinations of wall materials and homogenisation pressure on the microencapsulation of green coffee oil by spray drying. Food Res. Int. 2014, 61, 132–143. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Mesa, M.D. Coffee By-Products. In Coffee: Chemistry, Quality and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: Oxfordshire, UK, 2019; ISBN 9781782620044. [Google Scholar]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In Vitro health promoting properties of antioxidant dietary fiber extracted from spent coffee (Coffee arabica L.) grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Aparicio García, N.; Velazquez Escobar, F.; San Andres, M.I.; Sanchez-Fortun, S.; Blanch, G.P.; Fernandez-Gomez, B.; Guisantes Batan, E.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef]
- Edwards, A.D.; Slater, N.K.H. Protection of live bacteria from bile acid toxicity using bile acid adsorbing resins. Vaccine 2009, 27, 3897–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, C.M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Yago, M.D.; Martínez-Burgos, M.A.; Martínez-Victoria, E.; Gil, Á.; Ramirez-Tortosa, M.C. Monounsaturated and ω-3 but not ω-6 polyunsaturated fatty acids improve hepatic fibrosis in hypercholesterolemic rabbits. Nutrition 2005, 21, 363–371. [Google Scholar] [CrossRef]
- Cabezos, P.A.; Vera, G.; Castillo, M.; Fernández-Pujol, R.; Martín, M.I.; Abalo, R. Radiological study of gastrointestinal motor activity after acute cisplatin in the rat. Temporal relationship with pica. Auton. Neurosci. 2008, 141, 54–65. [Google Scholar] [CrossRef]
- Abalo, R.; Cabezos, P.A.; López-Miranda, V.; Vera, G.; González, C.; Castillo, M.; Fernández-Pujol, R.; Martín, M.I. Selective lack of tolerance to delayed gastric emptying after daily administration of WIN 55,212-2 in the rat. Neurogastroenterol. Motil. 2009, 21, 22–24. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A Review on Mycotoxins in Food and Feed: Malaysia Case Study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Garcia-Moraleja, A.; Font, G.; Manes, J.; Ferrer, E. Analysis of mycotoxins in coffee and risk assessment in Spanish adolescents and adults. Food Chem. Toxicol. 2015, 86, 225–233. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 123/2005 of 26 January 2005 amending Regulation (EC) No 466/2001 as regards ochratoxin A. Off. J. Eur. Union 2005, 2005, 2004–2006. [Google Scholar]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Spent coffee grounds as a renewable source of bioactive compounds. J. Biobased Mater. Bioenergy 2013, 7, 420–428. [Google Scholar] [CrossRef]
- De Roos, B.; Meyboom, S.; Kosmeijer-Schuil, T.G.; Katan, M.B. Absorption and urinary excretion of the coffee diterpenes cafestol and kahweol in healthy ileostomy volunteers. J. Intern. Med. 1998, 244, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Van Cruchten, S.T.J.; De Waart, D.R.; Kunne, C.; Hooiveld, G.J.E.J.; Boekschoten, M.V.; Katan, M.B.; Oude Elferink, R.P.J.; Witkamp, R.F. Absorption, distribution, and biliary excretion of cafestol, a potent cholesterol-elevating compound in unfiltered coffees, in mice. Drug Metab. Dispos. 2010, 38, 635–640. [Google Scholar] [CrossRef]
- Eren, F.H.; Besler, H.T. A 4-week consumption of light or dark roast unfiltered (Turkish) coffee affects cardiovascular risk parameters of homocysteine and cholesterol concentrations in healthy subjects: A randomized crossover clinical trial. Prog. Nutr. 2019, 21, 164–173. [Google Scholar] [CrossRef]
- Tirosh, O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell. Longev. 2018, 2018, 2548154. [Google Scholar] [CrossRef]
- Nieber, K. The Impact of Coffee on Health Author Pharmacokinetics and Mode of Action Bioactive Components in Coffee. Planta Med. 2017, 83, 1256–1263. [Google Scholar]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Muller-Lissner, S.A.; Kamm, M.A.; Scarpignato, C.; Wald, A. Myths and Misconceptions About Chronic Constipation. Am. J. Gastroenterol. 2005, 100, 232–242. [Google Scholar] [CrossRef] [PubMed]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Organs | Control | Treatment |
|---|---|---|
| Heart | 1.02 ± 0.23 a | 0.90 ± 0.07 a |
| Lungs | 1.90 ± 0.25 a | 1.56 ± 0.23 a |
| Liver | 9.72 ± 1.21 a | 9.94 ± 1.25 a |
| Kidneys | 2.08 ± 0.11 a | 2.00 ± 0.19 a |
| Spleen | 0.62 ± 0.15 a | 0.60 ± 0.10 a |
| Thymus | 0.66 ± 0.15 a | 0.58 ± 0.13 a |
| Adrenal glands | 0.16 ± 0.09 a | 0.18 ±0.08 a |
| Uterus | 1.52 ± 0.67 a | 1.36 ± 0.18 a |
| Brain | 1.80 ± 0.18 a | 1.70 ± 0.20 a |
| Analysis | SCGs | Bioaccessible Fraction | Fiber Fraction | |||
|---|---|---|---|---|---|---|
| Total Fat (%) | 21.79 ± 0.23 b | 11.87 ± 1.66 a | 14.21 ± 0.82 a | |||
| Fatty Acid | (mg/g) | (%) | (mg/g) | (%) | (mg/g) | (%) |
| C14:0 | 0.21 ± 0.01 c | 0.11 | 0.12 ± 0.01 a | 0.13 | 0.19 ± 0.01 b | 0.17 |
| C15:0 | 0.09 ± 0.00 b | 0.05 | 0.06 ± 0.00 a | 0.06 | 0.11 ± 0.01 c | 0.10 |
| C16:0 | 62.58 ± 2.38 c | 31.58 | 15.18 ± 0.94 a | 15.59 | 41.56 ± 0.74 b | 37.87 |
| C16:1n7 | 0.14 ± 0.01 a | 0.07 | 0.39 ± 0.03 c | 0.40 | 0.21 ± 0.01 b | 0.19 |
| C17:0 | 0.17 ± 0.01 b | 0.09 | 0.11 ± 0.01 a | 0.11 | 0.28 ± 0.01 c | 0.26 |
| C18:0 | 14.76 ± 0.56 b | 7.45 | 3.67 ± 0.30 a | 3.77 | 11.67 ± 0.16 c | 10.64 |
| C18:1n7c | 0.77 ± 0.03 b | 0.39 | 0.88 ± 0.06 c | 0.90 | 0.54 ± 0.03 a | 0.50 |
| C18:1n9c | 20.38 ± 0.84 c | 10.28 | 16.42 ± 1.23 b | 16.84 | 10.62 ± 0.43 a | 9.67 |
| C18:2n6c | 87.36 ± 3.73 c | 44.07 | 54.01 ± 4.82 b | 55.34 | 36.85 ± 0.99 a | 33.57 |
| C18:3n3 | 1.75 ± 0.09 c | 0.88 | 1.03 ± 0.09 b | 1.05 | 0.76 ± 0.03 a | 0.69 |
| C18:3n6 | n.d. | 0.00 | 0.08 ± 0.01 b | 0.08 | 0.04 ± 0.01 a | 0.04 |
| C20:0 | 6.68 ± 0.26 c | 3.37 | 1.23 ± 0.10 a | 1.26 | 3.90 ± 0.07 b | 3.55 |
| C20:1n9 | 0.83 ± 0.04 c | 0.42 | 0.44 ± 0.03 b | 0.45 | 0.32 ± 0.01 a | 0.30 |
| C20:2n6 | 0.12 ± 0.01 b | 0.06 | 0.17 ± 0.01 c | 0.17 | 0.09 ± 0.01 a | 0.08 |
| C20:3n6 | 0.21 ± 0.01 c | 0.10 | 0.04 ± 0.00 a | 0.04 | 0.13 ± 0.00 b | 0.12 |
| C20:4n6 | n.d. | 0.00 | 2.59 ± 0.37 b | 2.65 | 0.99 ± 0.10 a | 0.90 |
| C20:5n3 | 0.14 ± 0.01 c | 0.07 | 0.11 ± 0.01 b | 0.11 | 0.06 ± 0.01 a | 0.05 |
| C21:0 | n.d. | 0.00 | 0.10 ± 0.01 b | 0.10 | 0.05 ± 0.01 a | 0.04 |
| C22:0 | 0.96 ± 0.03 c | 0.49 | 0.20 ± 0.02 a | 0.21 | 0.59 ± 0.01 b | 0.54 |
| C22:1n9 | 0.21 ± 0.01 b | 0.11 | 0.10 ± 0.01 a | 0.11 | 0.10 ± 0.01 a | 0.09 |
| C22:4n6 | n.d. | 0.00 | 0.11 ± 0.02 b | 0.11 | 0.05 ± 0.00 a | 0.04 |
| C22:5n3 | n.d. | 0.00 | 0.20 ± 0.03 b | 0.21 | 0.10 ± 0.01 a | 0.09 |
| C22:6n3 | n.d. | 0.00 | 0.10 ± 0.01 b | 0.10 | 0.04 ± 0.01 a | 0.04 |
| C23:0 | 0.25 ± 0.00 c | 0.13 | 0.06 ± 0.01 a | 0.07 | 0.17 ± 0.01 b | 0.15 |
| C24:0 | 0.58 ± 0.02 c | 0.29 | 0.13 ± 0.02 a | 0.13 | 0.35 ± 0.00 b | 0.32 |
| SFA (%) | 43.54 ± 0.11 c | - | 21.43 ± 0.75 a | 53.64 ± 0.49 b | ||
| MUFA (%) | 11.27 ± 0.01 b | - | 18.70 ± 0.22 c | 10.74 ± 0.21 a | ||
| PUFA (%) | 45.19 ± 0.11 b | - | 59.87 ± 0.97 c | 35.62 ± 0.29 a | ||
| Diterpenes (µg/g) | ||||||
| Cafestol | 3095.39 ± 518.81 b | 414.39 ± 25.80 a | 1029.90 ± 55.51 a | |||
| Kahweol | 64.19 ± 9.35 b | 7.09 ± 1.68 a | 22.50 ± 0.34 a | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo-DeHond, A.; Cornejo, F.S.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Alonso, S.G.; Andres, M.I.S.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients 2019, 11, 1411. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061411
Iriondo-DeHond A, Cornejo FS, Fernandez-Gomez B, Vera G, Guisantes-Batan E, Alonso SG, Andres MIS, Sanchez-Fortun S, Lopez-Gomez L, Uranga JA, et al. Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients. 2019; 11(6):1411. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061411
Chicago/Turabian StyleIriondo-DeHond, Amaia, Fresia Santillan Cornejo, Beatriz Fernandez-Gomez, Gema Vera, Eduardo Guisantes-Batan, Sergio Gomez Alonso, Manuel Ignacio San Andres, Sebastian Sanchez-Fortun, Laura Lopez-Gomez, Jose Antonio Uranga, and et al. 2019. "Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds" Nutrients 11, no. 6: 1411. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11061411