The Effects of Caffeine on Metabolomic Responses to Muscle Contraction in Rat Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Muscle Treatment
2.3. Metabolomic Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Pattern Recognition of Metabolites
3.2. Discovery of Differentiating Metabolites
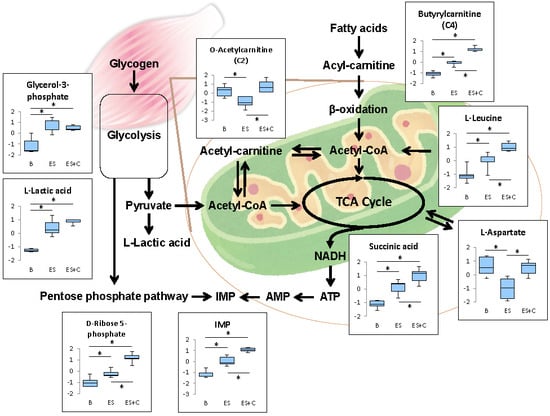
3.3. Pentose Phosphate Pathway/Nucleotide Synthesis Pathway
3.4. Acylcarnitine/Tricarboxylic Acid (TCA) Cycle
3.5. Amino Acid/Amino Acid Metabolism
3.6. Others
3.7. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Egawa, T.; Hamada, T.; Kameda, N.; Karaike, K.; Ma, X.; Masuda, S.; Iwanaka, N.; Hayashi, T. Caffeine acutely activates 5′adenosine monophosphate–activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metab. Clin. Exp. 2009, 58, 1609–1617. [Google Scholar] [CrossRef]
- Egawa, T.; Hamada, T.; Ma, X.; Karaike, K.; Kameda, N.; Masuda, S.; Iwanaka, N.; Hayashi, T. Caffeine activates preferentially alpha1-isoform of 5’amp-activated protein kinase in rat skeletal muscle. Acta Physiol. 2011, 201, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Egawa, T.; Kitani, K.; Oshima, R.; Ma, X.; Hayashi, T. Caffeine and contraction synergistically stimulate 5′-AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. Physiol. Rep. 2015, 3, e12592. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.E.; Rose, A.J.; Hellsten, Y.; Wojtaszewski, J.F.; Richter, E.A. Caffeine-induced ca(2+) release increases ampk-dependent glucose uptake in rodent soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E286–E292. [Google Scholar] [CrossRef] [PubMed]
- Raney, M.A.; Turcotte, L.P. Evidence for the involvement of camkii and ampk in ca2+-dependent signaling pathways regulating fa uptake and oxidation in contracting rodent muscle. J. Appl. Physiol. 2008, 104, 1366–1373. [Google Scholar] [CrossRef]
- Lally, J.S.V.; Jain, S.S.; Han, X.X.; Snook, L.A.; Glatz, J.F.C.; Luiken, J.J.F.P.; McFarlan, J.; Holloway, G.P.; Bonen, A. Caffeine-stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol. 2012, 205, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Westerblad, H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J. Physiol. 1995, 487, 331–342. [Google Scholar] [CrossRef]
- Konishi, M.; Kurihara, S. Effects of caffeine on intracellular calcium concentrations in frog skeletal muscle fibres. J. Physiol. 1987, 383, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.C.; Geiger, P.C.; Han, D.H.; Jones, T.E.; Holloszy, J.O. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007, 282, 18793–18799. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.G.; Brooks, M.B.; Cincotta, J.; Manjourides, J.D. Establishing a relationship between the effect of caffeine and duration of endurance athletic time trial events: A systematic review and meta-analysis. J. Sci. Med. Sport 2019, 22, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.G.; Morales, A.P.; Sampaio-Jorge, F.; Tinoco, F.D.S.; De Matos, A.A.; Leite, T.C. Acute effects of caffeine intake on athletic performance: A systematic review and meta-analysis. Rev. Chil. Nutr. 2017, 44, 283. [Google Scholar] [CrossRef]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Dotzert, M.S.; Murray, M.R.; McDonald, M.W.; Olver, T.D.; Velenosi, T.J.; Hennop, A.; Noble, E.G.; Urquhart, B.L.; Melling, C.W.J. Metabolomic Response of Skeletal Muscle to Aerobic Exercise Training in Insulin Resistant Type 1 Diabetic Rats. Sci. Rep. 2016, 6, 26379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starnes, J.W.; Parry, T.L.; O’Neal, S.K.; Bain, J.R.; Muehlbauer, M.J.; Honcoop, A.; Ilaiwy, A.; Christopher, P.M.; Patterson, C.; Willis, M.S. Exercise-Induced Alterations in Skeletal Muscle, Heart, Liver, and Serum Metabolome Identified by Non-Targeted Metabolomics Analysis. Metabolites 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Nesher, R.; Karl, I.E.; Kaiser, K.E.; Kipnis, D.M. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am. J. Physiol. 1980, 239, E454–E460. [Google Scholar] [CrossRef]
- Zetan, N.; Wallberg-Henriksson, H.; Henriksson, J. The rat epitrochlearis muscle: Metabolic characteristics. Acta Physiol. Scand. 1988, 134, 155–156. [Google Scholar] [CrossRef]
- Miyamoto, L.; Egawa, T.; Oshima, R.; Kurogi, E.; Tomida, Y.; Tsuchiya, K.; Hayashi, T. AICAR stimulation metabolome widely mimics electrical contraction in isolated rat epitrochlearis muscle. Am. J. Physiol. Physiol. 2013, 305, C1214–C1222. [Google Scholar] [CrossRef] [Green Version]
- Larsson, O.; Wahlestedt, C.; Timmons, J.A. Considerations when using the significance analysis of microarrays (SAM) algorithm. BMC Bioinform. 2005, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Gruning, N.M.; Kruger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.L.; Johnson, C.A.; Fernholz, K.; Cyr, J.A. The role of ribose in human skeletal muscle metabolism. Med. Hypotheses 2004, 62, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Richter, E.A.; Kiens, B.; Bangsbo, J. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J. Physiol. 1999, 520, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.E.; Hiebert, J.B.; Thimmesch, A.; Pierce, J.T.; Vacek, J.L.; Clancy, R.L.; Sauer, A.J.; Pierce, J.D. Understanding D-Ribose and Mitochondrial Function. Adv. Biosci. Clin. Med. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Palić, I.R.; Đorđević, A.S.; Ickovski, J.D.; Kostic, D.A.; Dimitrijevic, D.S.; Stojanović, G.S. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 1–8. [Google Scholar] [Green Version]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Furuichi, Y.; Goto-Inoue, N.; Fujii, N.L. Role of carnitine acetylation in skeletal muscle. J. Phys. Fit. Sports Med. 2014, 3, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Pan, D.A.; Hardie, G. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002, 30, 1064–1070. [Google Scholar] [CrossRef]
- Miura, S.; Tadaishi, M.; Kamei, Y.; Ezaki, O. Mechanisms of exercise- and training-induced fatty acid oxidation in skeletal muscle. J. Phys. Fit. Sports Med. 2014, 3, 43–53. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal Muscle Lipid Metabolism in Exercise and Insulin Resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [Green Version]
- Overmyer, K.A.; Evans, C.R.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, D.J.; Coon, J.J.; et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef]
- Huffman, K.M.; Koves, T.R.; Hubal, M.J.; Abouassi, H.; Beri, N.; Bateman, L.A.; Stevens, R.D.; Ilkayeva, O.R.; Hoffman, E.P.; Muoio, D.M.; et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 2014, 57, 2282–2295. [Google Scholar] [CrossRef] [Green Version]
- Seiler, S.E.; Koves, T.R.; Gooding, J.R.; Wong, K.E.; Stevens, R.S.; Ilkayeva, O.R.; Wittmann, A.H.; DeBalsi, K.L.; Davies, M.N.; Lindeboom, L.; et al. Carnitine Acetyltransferase Mitigates Metabolic Inertia and Muscle Fatigue During Exercise. Cell Metab. 2015, 22, 65–76. [Google Scholar] [CrossRef]
- Wagenmakers, A.J.M. 11 muscle amino acid metabolism at rest and during exercise: Role in human physiology and metabolism. Exerc. Sport Sci. Rev. 1998, 26, 287–314. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- She, P.; Zhou, Y.; Zhang, Z.; Griffin, K.; Gowda, K.; Lynch, C.J. Disruption of bcaa metabolism in mice impairs exercise metabolism and endurance. J. Appl. Physiol. 2010, 108, 941–949. [Google Scholar] [CrossRef]
- Trudeau, F. Aspartate as an Ergogenic Supplement. Sports Med. 2008, 38, 9–16. [Google Scholar] [CrossRef]
- Ahlboro, B.; Ekelund, L.-G.; Nilsson, C.-G. Effect of Potassium-Magnesium-Aspartate on the Capacity for Prolonged Exercise in Man. Acta Physiol. Scand. 1968, 74, 238–245. [Google Scholar] [CrossRef]
- de Haan, A.; van Doorn, J.E.; Westra, H.G. Effects of potassium + magnesium aspartate on muscle metabolism and force development during short intensive static exercise. Int. J. Sports Med. 1985, 6, 44–49. [Google Scholar] [CrossRef]
- Scislowski, P.W.D.; Hokland, B.M.; Thienen, W.I.A.D.-V.; Bremer, J.; Davis, E.J. Methionine metabolism by rat muscle and other tissues. Occurrence of a new carnitine intermediate. Biochem. J. 1987, 247, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Ishikura, K.; Ra, S.-G.; Ohmori, H. Exercise-induced changes in amino acid levels in skeletal muscle and plasma. J. Phys. Fit. Sports Med. 2013, 2, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Derungs, A.; Donzelli, M.; Berger, B.; Noppen, C.; Krahenbuhl, S.; Haschke, M. Effects of cytochrome p450 inhibition and induction on the phenotyping metrics of the basel cocktail: A randomized crossover study. Clin. Pharmacokinet. 2016, 55, 79–91. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine and exercise: Metabolism, endurance and performance. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef]
- Rossi, R.; Bottinelli, R.; Sorrentino, V.; Reggiani, C. Response to caffeine and ryanodine receptor isoforms in mouse skeletal muscles. Am. J. Physiol. Physiol. 2001, 281, C585–C594. [Google Scholar] [CrossRef]
- Huddart, H.; Abram, R.G. Modification of excitation-contraction coupling in locust skeletal muscle induced by caffeine. J. Exp. Zool. 1969, 171, 49–58. [Google Scholar] [CrossRef]
- Weber, A.; Herz, R. The Relationship between Caffeine Contracture of Intact Muscle and the Effect of Caffeine on Reticulum. J. Gen. Physiol. 1968, 52, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Luttgau, H.C.; Oetliker, H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J. Physiol. 1968, 194, 51–74. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef]








| Name | d Value | SD | p Value | q Value | |
|---|---|---|---|---|---|
| 1 | Theobromine | 5.7247 | 0.031023 | 0.0 | 0.0 |
| 2 | Butyrylcarnitine (C4) | 5.4855 | 0.055814 | 0.0 | 0.0 |
| 3 | IMP | 5.0676 | 0.10169 | 0.0 | 0.0 |
| 4 | β-Aminoisobutyric acid | 4.7254 | 0.14193 | 0.0 | 0.0 |
| 5 | L-Lactic acid | 4.6827 | 0.14713 | 0.0 | 0.0 |
| 6 | Cytosine | 4.4855 | 0.1717 | 5.4348 × 10−5 | 1.1445 × 10−4 |
| 7 | D-Ribose 5-phosphate | 4.4111 | 0.1812 | 5.4348 × 10−5 | 1.1445 × 10−4 |
| 8 | D-Glyceraldehyde | 4.3854 | 0.18452 | 5.4348 × 10−5 | 1.1445 × 10−4 |
| 9 | Hypoxanthine | 4.3489 | 0.18926 | 5.4348 × 10−5 | 1.1445 × 10−4 |
| 10 | Maltotriose | 4.2295 | 0.205 | 5.4348 × 10−5 | 1.1445 × 10−4 |
| 11 | D-Glucosamine 6-phosphate | 4.1269 | 0.21883 | 1.087 × 10−4 | 1.7607 × 10−4 |
| 12 | Choline | 4.1237 | 0.21927 | 1.087 × 10−4 | 1.7607 × 10−4 |
| 13 | Succinic acid | 4.0087 | 0.2351 | 1.087 × 10−4 | 1.7607 × 10−4 |
| 14 | N4-Acetylcytidine | 3.8995 | 0.25048 | 1.6304 × 10−4 | 2.4524 × 10−4 |
| 15 | L-Glutamate | 3.6907 | 0.28084 | 3.2609 × 10−4 | 4.2917 × 10−4 |
| 16 | Acetylenedicarboxylate | 3.6613 | 0.28523 | 3.2609 × 10−4 | 4.2917 × 10−4 |
| 17 | L-Leucine | 3.5719 | 0.29872 | 4.8913 × 10−4 | 5.7223 × 10−4 |
| 18 | 1-Myristoylglycerophosphocholine | 3.5708 | 0.29889 | 4.8913 × 10−4 | 5.7223 × 10−4 |
| 19 | L-Hexanoyl-carnitine (C6) | 3.4897 | 0.31135 | 5.4348 × 10−4 | 6.0234 × 10−4 |
| 20 | Serotonin | 3.4538 | 0.31693 | 5.9783 × 10−4 | 6.2945 × 10−4 |
| 21 | Threonate | 3.4109 | 0.32367 | 7.0652 × 10−4 | 7.0847 × 10−4 |
| 22 | Glycerol-3-phosphate | 3.2018 | 0.35736 | 0.001413 | 0.0012937 |
| 23 | 2-Oxobutanoate | 3.1997 | 0.35772 | 0.001413 | 0.0012937 |
| 24 | ATP | 3.009 | 0.38981 | 0.0021739 | 0.0018047 |
| 25 | N-Acetyl-L-alanine | 3.0039 | 0.3907 | 0.0021739 | 0.0018047 |
| 26 | L-Alanine | 2.9853 | 0.39391 | 0.0022283 | 0.0018047 |
| 27 | Xanthine | 2.9261 | 0.40421 | 0.0023913 | 0.001865 |
| 28 | Imidazole lactic acid | 2.913 | 0.40651 | 0.0025543 | 0.001921 |
| 29 | N8-Acetylspermidine | 2.8766 | 0.41292 | 0.002663 | 0.0019337 |
| 30 | NADP | 2.8527 | 0.41718 | 0.0029348 | 0.0020305 |
| 31 | Oxypurinol | 2.8504 | 0.41759 | 0.0029891 | 0.0020305 |
| 32 | Taurine | 2.7011 | 0.44464 | 0.0040761 | 0.0026823 |
| 33 | O-Acetylcarnitine (C2) | 2.6096 | 0.46169 | 0.0052174 | 0.0032651 |
| 34 | Isatin | 2.5882 | 0.46572 | 0.0052717 | 0.0032651 |
| 35 | L-Fucose | 2.5586 | 0.47133 | 0.0057065 | 0.0034334 |
| 36 | Spermidine | 2.5126 | 0.48016 | 0.0064674 | 0.0037831 |
| 37 | 1-Palmitoleoylglycerophosphocholine | 2.4683 | 0.48872 | 0.0071739 | 0.0040357 |
| 38 | GDP | 2.4586 | 0.49062 | 0.0072826 | 0.0040357 |
| 39 | Iminodiacetate | 2.3301 | 0.51606 | 0.0096196 | 0.0051941 |
| 40 | L-Methionine | 2.3125 | 0.51962 | 0.010054 | 0.0052477 |
| 41 | γ-Glu-leu | 2.3104 | 0.52004 | 0.010217 | 0.0052477 |
| 42 | Maleamate | 2.2816 | 0.52587 | 0.010761 | 0.0052964 |
| 43 | L-Aspartate | 2.2673 | 0.52879 | 0.010815 | 0.0052964 |
| 44 | Guanidinosuccinic acid | 2.2214 | 0.53822 | 0.011793 | 0.0056442 |
| 45 | L-Valine | 2.169 | 0.54911 | 0.013478 | 0.0062447 |
| 46 | CDP | 2.1582 | 0.55136 | 0.013641 | 0.0062447 |
| 47 | EDTA | 2.134 | 0.55645 | 0.014511 | 0.0064614 |
| 48 | 2-Hydroxyisobutyric acid | 2.126 | 0.55815 | 0.014728 | 0.0064614 |
| 49 | Creatinine | 2.1072 | 0.56212 | 0.015163 | 0.0065164 |
| 50 | 3-Methyl-2-oxobutyric acid | 2.0385 | 0.57683 | 0.017065 | 0.0071872 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, S.; Hayashi, T.; Egawa, T. The Effects of Caffeine on Metabolomic Responses to Muscle Contraction in Rat Skeletal Muscle. Nutrients 2019, 11, 1819. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081819
Tsuda S, Hayashi T, Egawa T. The Effects of Caffeine on Metabolomic Responses to Muscle Contraction in Rat Skeletal Muscle. Nutrients. 2019; 11(8):1819. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081819
Chicago/Turabian StyleTsuda, Satoshi, Tatsuya Hayashi, and Tatsuro Egawa. 2019. "The Effects of Caffeine on Metabolomic Responses to Muscle Contraction in Rat Skeletal Muscle" Nutrients 11, no. 8: 1819. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081819