A 5Ad Dietary Protocol for Functional Bowel Disorders
Abstract
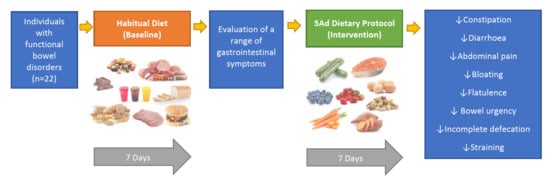
:1. Introduction
- √
- Provide a dietary approach that is less restrictive, uncomplex, nutritionally adequate, and suitable for long-term adherence.
- √
- Produce a universal dietary approach for functional bowel disorders (FBDs), effective in reducing, not only abdominal symptoms, but the wide range of complaints associated with all forms and severities of FBDs, including diarrhoea- and constipation-related symptoms.
- √
- Remove the need to distinguish between each FBD when considering dietary management for symptom relief.
- √
- Adopt a “bottom-up” approach to exclude only foods with a large amount of known offending food components (e.g., oligosaccharides, resistant proteins, food additives, and highly processed/refined foods).
- √
- Use of five simple food groups from which at least one item should be consumed per day to ensure a balanced and complementary diet.
- √
- Advise the consumption of around 1 kg fruit/vegetables per day to ensure adequate dietary fibre intake.
- √
- Include only fruits with equimolar concentration of fructose and glucose.
- √
- Include low-lactose dairy products to ensure adequate calcium intakes.
- √
- Focus on foods which can be consumed rather than a list of foods to avoid.
- √
- Use all-natural whole foods to avoid the need to purchase unhealthy and costly commercial gluten-free or low fermentable oligo-, di-, mono-saccharides and polyols (FODMAP) alternatives.
- √
- Provide a universal approach for all forms and severities of functional bowel symptoms, not only those meeting the irritable bowel syndrome (IBS) diagnostic criteria, to remove the difficulties associated with distinguishing between each functional bowel disorder (FBD).
- √
- Provide an easy-to-administer, simple-to-follow, and long-term approach.
2. Population and Study Design
- √
- 18 years or over.
- √
- Not pregnant (if applicable).
- √
- Experience abdominal pain and at least 2 of the following symptoms: chronic constipation, diarrhoea, or an alternation of both, abdominal bloating, flatulence/excessive gas, bowel urgency, straining, or incomplete defecation.
- √
- These symptoms must be present for ≥3 times per week, with symptom onset occurring ≥1 year prior to participation.
- √
- No known underlying pathology (e.g., Crohn’s disease, ulcerative colitis, celiac disease), self-reported.
- √
- No gastrointestinal surgery within the past year.
- √
- Non-vegans (vegetarians and pescatarians included if consume eggs and/or dairy).
- √
- Those taking prescribed medications which may affect bowel function included if the intake is maintained throughout the baseline and intervention period.
3. Results
3.1. Participants and Baseline Characteristics
3.2. Outcome Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Whitehead, W.E.; Toner, B.B.; Diamant, N.; Hu, Y.J.B.; Bangdiwala, S.I.; Jia, H. What determines severity among patients with painful functional bowel disorders? Am. J. Gastroenterol. 2000, 95, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed] [Green Version]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Simren, M.; Palsson, O.S.; Whitehead, W.E. Update on Rome IV Criteria for Colorectal Disorders: Implications for Clinical Practice. Curr. Gastroenterol. Rep. 2017, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, M.A.; Spiegel, B.M.R.; Chang, L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment. Pharmacol. Ther. 2010, 32, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Effect of Gender on Prevalence of Irritable Bowel Syndrome in the Community: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Soubieres, A.; Wilson, P.; Poullis, A.; Wilkins, J.; Rance, M. Burden of irritable bowel syndrome in an increasingly cost-aware National Health Service. Front. Gastroenterol. 2015, 6, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, M.; Whorwell, P. The global impact of IBS: Time to think about IBS-specific models of care? Ther. Adv. Gastroenterol. 2017, 10, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Suares, N.C.; Ford, A.C. Prevalence of, and Risk Factors for, Chronic Idiopathic Constipation in the Community: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Heidelbaugh, J.J.; Stelwagon, M.; Miller, S.A.; Shea, E.P.; Chey, W.D. The Spectrum of Constipation-Predominant Irritable Bowel Syndrome and Chronic Idiopathic Constipation: US Survey Assessing Symptoms, Care Seeking, and Disease Burden. Am. J. Gastroenterol. 2015, 110, 580–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.K.; Palsson, O.S.; Turner, M.J.; Levy, R.L.; Feld, A.D.; Von Korff, M.; Whitehead, W.E. Inability of the Rome III Criteria to Distinguish Functional Constipation from Constipation Subtype Irritable Bowel Syndrome. Am. J. Gastroenterol. 2010, 105, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Devroede, G.; Bon, C.; Bejou, B.; Mary, F.; Benamouzig, R. Is-it possible to distinguish irritable bowel syndrome with constipation from functional constipation? Tech. Coloproctol. 2017, 21, 125–132. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Food Choice as a Key Management Strategy for Functional Gastrointestinal Symptoms. Am. J. Gastroenterol. 2012, 107, 657–666. [Google Scholar] [CrossRef]
- Kanazawa, M.; Fukudo, S. Effects of fasting therapy on irritable bowel syndrome. Int. J. Behav. Med. 2006, 13, 214–220. [Google Scholar] [CrossRef]
- Spencer, M.; Chey, W.D.; Eswaran, S. Dietary Renaissance in IBS: Has Food Replaced Medications as a Primary Treatment Strategy? Curr. Treat. Options Gastroenterol. 2014, 12, 424–440. [Google Scholar] [CrossRef]
- Tuck, C.J.; Vanner, S.J. Dietary therapies for functional bowel symptoms: Recent advances, challenges, and future directions. Neurogastroenterol. Motil. 2018, 30, e13238. [Google Scholar] [CrossRef]
- Halmos, E.P. When the low FODMAP diet does not work. J. Gastroenterol. Hepatol. 2017, 32, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Antoine, J.M.; Azpiroz, F.; Bourdet-Sicard, R.; Brandtzaeg, P.; Calder, P.C.; Gibson, G.R.; Guarner, F.; Isolauri, E.; Pannemans, D.; et al. PASSCLAIM—Gut health and immunity. Eur. J. Nutr. 2004, 43 (Suppl. 2), ii118–ii173. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D.; Burley, V. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low FODMAP diet in the management of irritable bowel syndrome: An evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet. 2018, 31, 239–255. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in fodmaps reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta- analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C.E. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef]
- Heaton, K.W.; Radvan, J.; Cripps, H.; Mountford, R.A.; Braddon, F.E.; Hughes, A.O. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut 1992, 33, 818–824. [Google Scholar] [CrossRef]
- De Vries, J.; Birkett, A.; Hulshof, T.; Verbeke, K.; Gibes, K. Effects of Cereal, Fruit and Vegetable Fibers on Human Fecal Weight and Transit Time: A Comprehensive Review of Intervention Trials. Nutrients 2016, 8, 130. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Basilisco, G.; De Marco, E.; Tomba, C.; Cesana, B.M. Bowel Urgency in Patients with Irritable Bowel Syndrome. Gastroenterology 2007, 132, 38–44. [Google Scholar] [CrossRef]
- Mangel, A.W.; Wang, J.; Sherrill, B.; Gnanasakthy, A.; Ervin, C.; Fehnel, S.E. Urgency as an Endpoint in Irritable Bowel Syndrome. Gastroenterol. Res. 2011, 4, 9–12. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Liu, H.-N.; Wu, H.; Chen, Y.-Z.; Chen, Y.-J.; Shen, X.-Z.; Liu, T.-T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Guo, Y.; Logan, H.L.; Glueck, D.H.; Muller, K.E. Selecting a sample size for studies with repeated measures. BMC Med. Res. Methodol. 2013, 13, 100. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Enck, P. Placebo effects and their determinants in gastrointestinal disorders. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 472–485. [Google Scholar] [CrossRef]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef]




| Baseline Characteristics | |
|---|---|
| Age range (mean ± SE) | 20–75 (49.29 ± 3.35) |
| Gender n, (%) | |
| Females | 16 (73%) |
| Males | 6 (27%) |
| BMI category n, (%) | |
| <18.5 kg/m² | 1 (5%) |
| 18.5–24.9 kg/m² | 13 (59%) |
| 25–29.9 kg/m² | 4 (18%) |
| ≥30 kg/m² | 4 (18%) |
| PAL n, (%) | |
| Sedentary | 1 (5%) |
| Light | 1 (5%) |
| Moderate | 16 (73%) |
| Vigorous | 4 (18%) |
| IBS diagnosed n, (%) | |
| Yes | 11 (50%) |
| No | 11 (50%) |
| Baseline average stool type n, (%) | |
| Constipation (type 1–2) | 8 (36%) |
| Normal (type 3–4) | 7 (32%) |
| Diarrhoea (type 5–7) | 7 (32%) |
| Baseline defecation frequency n, (%) | |
| Low (<6 per week) | 5 (23%) |
| Medium (6–10 per week) | 5 (23%) |
| High (>10 per week) | 12 (54%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, F.; Stribling, P. A 5Ad Dietary Protocol for Functional Bowel Disorders. Nutrients 2019, 11, 1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081938
Ibrahim F, Stribling P. A 5Ad Dietary Protocol for Functional Bowel Disorders. Nutrients. 2019; 11(8):1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081938
Chicago/Turabian StyleIbrahim, Fandi, and Philippa Stribling. 2019. "A 5Ad Dietary Protocol for Functional Bowel Disorders" Nutrients 11, no. 8: 1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11081938