1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide [
1]. Diet and lifestyle are closely associated with CRC. Previous studies pointed out that dietary pattern could significantly affect the risk of cancer [
2]. Western diet, a diet high in fat and low in dietary fiber, increases the risk of CRC [
3].
The development of CRC is a multiple and long-term process, including initiation, promotion, progression, and invasion. Inflammation and oxidative stress may initiate and promote CRC development. Cyclooxygenase-2 (COX-2) mediates inflammation and plays an important role in CRC. Increased COX-2 expression has been detected in both colorectal tumor-bearing animals [
4] and CRC patients [
5].
Overexpression of COX-2 can inhibit apoptosis, including both intrinsic and extrinsic pathways [
6]. In CRC cells, reduction of B-cell lymphoma 2 (Bcl-2) protein expression and enhancement of Bcl-2-associated X (Bax) protein expression significantly inhibited cancer cell proliferation [
7]. In addition, upregulation of caspase-3, caspase-8, and caspase-9 could promote apoptosis in HCT-116 cells [
8]. These studies suggest that the increased expression of pro-apoptotic proteins and the decreased expression of anti-apoptotic proteins play a key role in the control of CRC.
Aberrant crypt foci (ACF), a precancerous lesion in the colon, have been generally accepted for use as a biomarker to investigate the effects of dietary factors on colon carcinogenesis in rats since it was first reported by Bird in 1987 [
9]. Intestinal goblet cells secrete two different mucins, the sulfomucin (SUM) and the sialomucin (SIM) [
10]. According to different mucin production, ACF can be classified into sulfomucin-producing ACF (SUM-ACF), sialomucin-producing ACF (SIM-ACF), and mucin-depleted foci (MDF). MDF with absent or scant secretion of mucin are positively correlated to the number of colonic tumors [
11]. MDF become larger over time and spread more in the distal colon, thus they are an indicator of precancerous lesions and can be used as a short-term endpoint for CRC studies [
12]. Therefore, ACF and their produced mucins can be used as good biomarkers to detect the efficacy of chemopreventive agents in colon carcinogenesis.
Djulis (
Chenopodium formosanum) is a native cereal crop planted in Taiwan and traditionally called “ruby of cereals” for its bright red color. Djulis has many health benefits, such as anti-adipogenesis [
13] and recovering liver injury [
14,
15]. Some studies found that the color of djulis was mainly from betalains, including betanin, isobetanin, amaranthine, and isoamaranthine, which were directly related to its antioxidant capacity [
16]. Moreover, djulis contains high levels of dietary fiber, proteins, and grain-limited essential amino acids (e.g., lysine) [
17].
Certain dietary fiber, as a good prebiotics, can promote the growth of the intestinal bacterial population towards a relative increase in
Bifidobacterium and/or
Lactobacillus species [
18]. Some studies have indicated that modulation of the gut microbiota positively affects the interaction between microbiota and the host immune system, and thus may be beneficial in suppressing CRC development [
19]. Moreover, emerging studies suggest that synbiotics, the combination of prebiotics and probiotics, are more effective in preventing CRC than either prebiotics or probiotics alone [
20].
Our previous study demonstrated that dietary djulis inhibited the development of precancerous lesions of CRC in a carcinogen-induced animal model [
21]. Besides, we also found that
Lactobacillus acidophilus reduced inflammation in lipopolysaccharide (LPS)- and tumor necrosis factor alpha (TNF-α)-induced inflammatory colon cancer cells [
22]. However, the effect of combination of djulis and probiotics and the detailed mechanism of action remain to be elucidated. The aim of this study was to investigate the preventive effect of djulis combined with
L. acidophilus on colon carcinogenesis in a rat model.
2. Materials and Methods
2.1. Materials
Djulis was acquired from Sinfong Agritech Co. (Taipei, Taiwan). L. acidophilus LA-5® powder was from Chr. Hansen (Hørsholm, Denmark). BD DifcoTM Lactobacilli MRS Broth was purchased from BDTM (Franklin Lakes, NJ, USA). Methylene blue, acetic acid, and iron (III) chloride hexahydrate were purchased from Nacalai Tesque Inc. (Tokyo, Japan), Showa Chemicals Co. (Tokyo, Japan) and Shimakyu Pure Chemicals (Osaka, Japan), respectively. Caspase-3 antibody (GTX110543) was purchased from Genetex Inc. (Irvine, CA, USA). Bax antibody (Catalogue number: 2772) and Bcl-2 antibody (Catalogue number: 2870) were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Proliferating cell nuclear antigen (PCNA) antibody (Catalogue number: 13110), Goat anti-rabbit IgG secondary antibody, peroxidase AffiniPure goat anti-mouse IgG, and COX-2 antibody (Catalogue number: ab6665) were purchased from Abcam (Cambridge, UK), Southern Biotechnology Associates, Inc. (Birmingham, AL, USA) and Jackson ImmunoResearch Inc. (West Grove, PA, USA), respectively. Antibody dilutions for caspase-3, Bax, Bcl-2, PNCA, and COX-2 were 1: 2000, 1:1000, 1:1000, 1:1000, and 1:5000, respectively. β-Actin antibody, 1,2-dimethylhydrazine (DMH), dextran sulfate sodium (DSS) salt from Leuconostoc, N, N′-dimethyl-m-phenylenediamine, Alcian blue, N, N′-dimethyl-p-phenylenediamine, agar, and the other chemicals were all purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Experimental Diets
The experimental diet was an AIN-93G-based diet containing djulis alone or in combination with
L. acidophilus. The content of
L. acidophilus LA-5
® in powder was 6 × 10
10 colony-forming unit (cfu)/g. Diets were stored at room temperature and then analyzed for the bacteria count [
23] to ensure that the doses of groups DLA and DHA were 5 × 10
6 cfu
L. acidophilus LA-5
®/g and 5 × 10
7 cfu
L. acidophilus LA-5
®/g, respectively (
Figure 1).
2.3. Animals and Treatments
The protocol used herein was approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University. The approval number is LAC-2015-0400. Sixty male F344 rats (3–5 weeks old) were from National Laboratory Animal Center (Taipei, Taiwan) and housed under standard conditions (21 ± 2 °C, 40–60% humidity under a 12-h light-dark cycle). The rats had free access to diet and water. After an adaptation period (1 week), the rats were randomized into 5 groups (12 rats per group) and fed B (AIN-93G, blank), C (AIN-93G, control), D (10% djulis), DLA (10% djulis plus 5 × 10
6 cfu/g of
L. acidophilus LA-5
®), and DHA (10% djulis plus 5 × 10
7 cfu/g of
L. acidophilus LA-5
®) diets, respectively (
Table 1). The composition of djulis and experimental diet are shown in
Table 2 and
Table 3, respectively. After one week of experimental diet feeding, rats in groups C, D, DLA, and DHA were given intraperitoneal injections of DMH (40 mg/kg) for 3 consecutive days during the second week of dietary treatment, and after DMH injection, these groups were treated with 3% DSS in drinking water for one week (
Figure 2). The fresh experimental diets were supplied every three days. Body weight and feed consumption were measured every three days during the experimental period. After 10 weeks of feeding, all rats were sacrificed and the cecum, colons, and feces were collected and examined for precancerous lesions and biomarkers.
2.4. Fecal L. acidophilus Counting
Serial dilutions of feces were conducted on Lactobacilli MRS Broth-agar. The count of fecal
L. acidophilus was determined on the above-mentioned medium [
24].
2.5. Measurement of the Cecum
The cecum was excised, weighed, and then split open [
25]. The weights of the cecum wall and content were also recorded.
2.6. Analysis of Colonic ACF
ACF were analyzed by the method described in our previous study [
26]. The colon was removed, opened longitudinally and rinsed in saline, and then fixed flat between filter papers in 10% buffered formalin for 24 h. After being stained with 0.2% methylene blue solution for 5 min, fixed sections were placed on microscopic slides and the mucosal side was examined under a light microscope (Nikon Corp., Tokyo, Japan) at 40× magnification. Total numbers of ACF and aberrant crypts (ACs) in each focus were counted and the colonic area was calculated by NIS-Elements microscope imaging software (Nikon Corp., Tokyo, Japan). All data of ACF and AC were presented as number/cm
2.
2.7. Determination of Mucin Production in ACF
The distal colons were immersed in 75% ethanol for fading after being stained with methylene blue, and then stained with high-iron diamine Alcian blue (HIDAB). The colons were observed under a light microscope (Nikon Corp., Tokyo, Japan) at 40× magnification. ACF stained bright or dark blue indicated SIM production while those stained dark brown indicated SUM production. The definition of SUM-ACF were samples with more than 85% SUM-producing cells, and for SIM-ACF were those with more than 85% SIM-producing cells. Those with not more than 85% SUM-producing or 85% SIM-producing cells were defined as mixed-type (MIX)-ACF. Furthermore, foci with very little or no secretion of any mucin were defined as MDF [
27]. The colonic area was calculated by NIS-Elements microscope imaging software (Nikon Corp., Tokyo, Japan). Data of SIM-, SUM-, MIX-ACF, and MDF were presented as number/cm
2.
2.8. Analysis of Protein Expression
Colon samples were homogenized in in five volumes of modified radioimmunoprecipitation assay (RIPA) buffer (0.5 M Tris-HCl, 2.5% deoxycholic acid, 1.5 M NaCl, 10% NP-40, 10 mM ethylenediaminetetraacetic acid, pH 7.4) and 10% protease inhibitor cocktail. The supernatants of homogenates were collected by centrifuging the homogenates at 10,000× g at 4 °C for 15 min. The total protein concentration of supernatants was confirmed by Bradford protein assay. Western blot was used for protein analysis. Protein samples were separated on 10% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin (BSA) in Tris-buffered saline containing 1% Tween 20 (TBST), the membranes were incubated with the appropriate antibodies at 4 °C for 16 h. The membranes were then washed with TBST and incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies for 1 h. After washing with TBST, the immunocomplexes were visualized by chemiluminescence reagents and detected by chemiluminometer (BioSpectrum AC Imaging System, Ultra-Violet Products Ltd., Cambridge, United Kingdom).
2.9. Statistical Analysis
Data were presented as means ± SD. Differences among the experimental data were assessed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. All statistical analyses were carried out using the SAS software (SAS Institute, Cary, NC, USA). All p values < 0.05 were considered statistically significant.
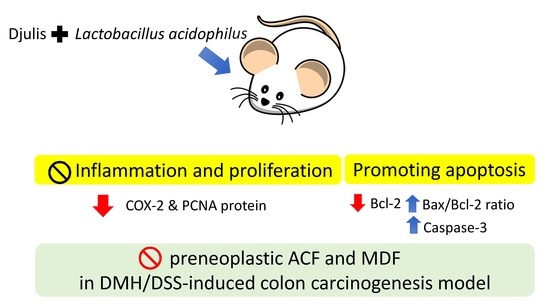
4. Discussion
Overall, the present study showed that dietary treatment with either djulis alone or in combination with L. acidophilus showed a preventive effect on DMH/DSS-induced colon carcinogenesis in rats. The preventive effects could be proven by the reduction of distal ACF, the low number of MDF, and the decreased expression of Bcl-2 in all djulis-treated groups. Moreover, the synbiotic combination of djulis and L. acidophilus showed better inhibitory effects on the formation of large ACF and the expressions of PCNA and COX-2, as well as a better enhancing effect on the Bax/Bcl-2 ratio and caspase-3 expression compared to djulis alone.
The model of DMH/DSS-induced inflammation-associated colon carcinogenesis in rats has been widely used in many studies. Animals are often given DSS shortly after injection of DMH, which causes a decrease in the secretion of SUM-type mucin in the mucosa, impaired intestinal barrier function, induction of intestinal inflammation, and acceleration of CRC development [
29]. In addition, as a promoter of CRC, DSS can significantly promote the formation of MDF [
30]. Therefore, we used DMH and DSS to induce colitis-associated carcinogenesis in this study. The ACF incidence rates of all DMH/DSS-treated groups were 100%, representing successfully induced colon carcinogenesis by the DMH/DSS treatment.
ACF is a precancerous lesion of CRC in rodents and humans, which may progress to early and advanced adenomas [
31,
32]. As the total number of colonic ACF increased, the risk of CRC also increased [
33]. In the present study, group DHA, which combined djulis with a high dose of
L. acidophilus, significantly reduced the total ACF number compared to group C. Besides, the number of ACs in ACF can also be a marker to evaluate the progression of CRC. A previous study indicated that the greater the number of large ACF, the greater the risk of tumorigenesis [
34]. In the present study, group C had the highest number of large ACF among groups, whereas the combined treatment of djulis and
L. acidophilus in groups DLA and DHA significantly reduced the numbers of large ACF. These results suggest the potential of djulis and
L. acidophilus in chemoprevention of CRC.
Several studies indicated that chemical carcinogens induced colon cancer progression primarily by causing damage to the colonic mucosa [
35]. ACF mainly appear in the distal colon at an early stage and expand to the proximal colon during advanced progression, and thus there is a relatively high number of ACF in the middle and distal colons [
36,
37]. A similar result was found in this study. ACF mainly distributed in the distal colon of all DMH/DSS-treated groups. Furthermore, both djulis alone and the combination of djulis with
L. acidophilus significantly decreased the ACF number in the distal colon. These findings demonstrate that djulis and
L. acidophilus have the ability to prevent colon carcinogenesis through inhibiting ACF development at an early stage.
Secretion of colonic mucins may change during the progression of CRC. A previous study indicated that secretion of mucin in ACF would gradually change from SUM to SIM [
38]. It was also mentioned in another study that SIM-ACF had a higher hyperplastic index in the crypt, more dysplasia, and larger abnormal crypts compared to SUM-ACF [
27]. In the present study, combined treatment of djulis and
L. acidophilus (groups D, DLA, and DHA) significantly reduced the number of SIM-ACF compared to group C. It suggests that either djulis alone or the combination of djulis with
L. acidophilus could inhibit the progress of CRC by regulating the colonic secretion of mucins.
A previous study found that both azoxymethane (AOM) and DMH promoted the formation of MDF in the colon [
39]. In the present study, groups D, DLA, and DHA had a significantly lower MDF number than group C. Moreover, group DHA had the lowest MDF incidence. MDF that exhibit absent or scant production of mucin carry alterations in cell signaling pathways and gene mutations, with a frequency similar to that observed in tumors [
40]. It has been proven that the number of MDF was positively correlated to the incidence of CRC [
41]. These results show that either djulis alone or the combination of djulis with
L. acidophilus have an inhibitory effect on the progression from primary to advanced precancerous lesions during colon carcinogenesis.
The present study showed that the fecal count of
L. acidophilus in group DHA was significantly higher than that in group C. Previous studies have shown that the induction by DMH and DSS will lead to an increase in the intestinal pH, and thus reduce the number of intestinal probiotics, such as
Lactobacillus and
Bifidobacterium [
42]. A similar phenomenon was found in patients with irritable bowel syndrome. After treatment with
L. acidophilus (10
9 cfu/day) and dietary fiber (consisting of 90% inulin and 10% oligofructose) for four weeks, the content of
L. acidophilus in the feces was significantly increased compared to the placebo group, which confirmed that synbiotics can modulate the intestinal microflora [
43]. A preliminary study in our laboratory demonstrated that the combination of probiotic
L. acidophilus and germinated brown rice rich in dietary fiber suppressed DMH/DSS-induced colonic precancerous lesions via regulation of antioxidation and apoptosis in rats [
44]. These findings suggest that such a synbiotic combination may be a potential functional food or chemopreventive agent for the control of CRC [
44]. Meanwhile, the present study proved that the combination of djulis and a high dose of
L. acidophilus improved DMH/DSS-induced reduction of
L. acidophilus in feces. Overall, it is speculated that djulis could provide prebiotic dietary fiber for promoting the growth of
L. acidophilus in the intestine.
The cecum is the main fermentation organ in rodents. Short-chain fatty acids (SCFAs) produced by fermentation can maintain intestinal epithelial cells, reduce intestinal pH, and stimulate the growth of probiotics [
45]. Studies showed that simultaneous administration of inulin and probiotics to mice increased the cecal weight, which represented a higher microbial content [
46]. Femia et al. [
47] pointed out that in an AOM-induced CRC rat model, the combination of inulin and probiotics (
Lactobacillus rhamnosus and
Bifidobacterium lactis) increased the concentration of SCFAs in the gut and inhibited the formation of ACF. Moreover, the synbiotic combination showed a stronger inhibitory effect on ACF than the probiotics or prebiotics alone [
47]. In the present study, the relative cecum content weight was negatively correlated to the total ACF number in the distal colon (
r = −0.51,
p < 0.05) and positively correlated to caspase-3 protein expression (
r = 0.79,
p < 0.0001). It suggests that synbiotics may inhibit the formation of colonic precancerous lesions by promoting fermentation in the cecum.
Chronic inflammation plays an important role in cancer development in several tissues, including the large intestine. The progression of CRC is accelerated by COX-2, which may promote cell proliferation, anti-apoptosis, invasion, metastasis, angiogenesis, and drug resistance in colon cancer [
48]. In the present study, the expression of inflammation-related COX-2 was increased by DMH/DSS treatment; conversely, COX-2 expression was reduced by either djulis alone or the combination of djulis with
L. acidophilus. Moreover, the combination of djulis with a high dose of
L. acidophilus showed the best inhibitory effect on COX-2 expression. These findings suggest that djulis and
L. acidophilus may suppress colon carcinogenesis via an inflammation-associated pathway.
Although djulis alone had no effect on proliferation-related PCNA, the combination of djulis with
L. acidophilus reduced PCNA expression in the present study. Besides, the number of SIM-ACF was positively correlated to PCNA expression (
r = 0.55,
p < 0.05). A previous study found that both inhibition of PCNA expression and promotion of caspase-3 expression were confirmed in the activation of the extrinsic pathway of apoptosis [
49]. Increasing the Bax/Bcl-2 ratio or inhibiting Bcl-2 expression, both markers of apoptosis, by treatment with bioactive compounds in colon cancer cells limited their growth [
50,
51]. The present study found that the combination of djulis with
L. acidophilus could increase the Bax/Bcl-2 ratio and caspase-3 expression while treatment with djulis alone had no such effect. It suggests that the combination of djulis with
L. acidophilus may inhibit cell proliferation and promote apoptosis in the DMH/DSS-induced CRC animal model.
In this study, 10% djulis in the experimental diet is equal to 44 g djulis for a 60 kg human per day. Besides, the doses of probiotics used in the DLA and DHA groups are equal to 2.5 × 109 and 2.5 × 1010 cfu L. acidophilus for a 60 kg human per day, respectively. It is easy to have these doses of probiotics from commercial yogurts, which usually contain 108 to 1010 cfu probiotics in each bottle. Therefore, the doses of djulis and L. acidophilus used in this study are feasible for humans to take in daily life.