Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence
Abstract
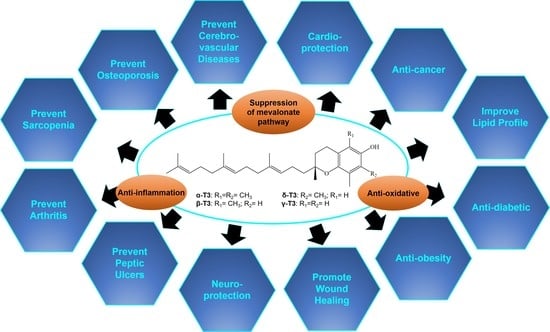
:1. Introduction
2. Pharmacokinetics of Tocotrienol
3. Effects of Tocotrienol on Lipid Metabolism
4. Effects of Tocotrienol on Cardiovascular Diseases
5. Effects of Tocotrienol on Cerebrovascular Diseases
6. Effects of Tocotrienol on Osteoporosis
7. Effects of Tocotrienol on Arthritis
8. Effects of Tocotrienol on Muscle Diseases
9. Effects of Tocotrienol on Peptic Ulcers
10. Effects of Tocotrienol on Neurodegenerative Diseases
11. Effects of Tocotrienol on Wound Healing
12. Effects of Tocotrienol on Obesity, Diabetes, and Their Complications
13. Effects of Tocotrienol on Cancers
14. Anti-Inflammatory Properties of Tocotrienol
15. Comparison between the Effects of Tocotrienol and Tocopherol
16. Safety of Tocotrienol and Tocopherol
17. Research Gaps and Future Perspective
18. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Camps, J.; Garcia-Heredia, A. Introduction: Oxidation and inflammation, a molecular link between non-communicable diseases. Adv. Exp. Med. Biol. 2014, 824. [Google Scholar] [CrossRef]
- Bessesen, D.; Hill, J.; Wyatt, H. Hormones and Obesity. J. Clin. Endocrinol. Metab. 2004, 89, E2. [Google Scholar] [CrossRef] [Green Version]
- Keizer, H.G. The “Mevalonate hypothesis”: A cholesterol-independent alternative for the etiology of atherosclerosis. Lipids Health Dis. 2012, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E As a Potential Interventional Treatment for Metabolic Syndrome: Evidence from Animal and Human Studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol is a cardioprotective agent against ageing-associated cardiovascular disease and its associated morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Mohamad, N.V.; Ibrahim, N.; Chin, K.Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.-Y.; Ima-Nirwana, S. The Role of Tocotrienol in Preventing Male Osteoporosis-A Review of Current Evidence. Int. J. Mol. Sci. 2019, 20, 1355. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.-Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis - A Review of the Current Evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khor, S.C.; Abdul Karim, N.; Ngah, W.Z.; Yusof, Y.A.; Makpol, S. Vitamin E in sarcopenia: Current evidences on its role in prevention and treatment. Oxid. Med. Cell Longev. 2014, 2014, 914853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.Y.; Tay, S.S. A Review on the Relationship between Tocotrienol and Alzheimer Disease. Nutrients 2018, 10, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, K.-L.; Chin, K.-Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular Mechanisms of Action of Tocotrienols in Cancer: Recent Trends and Advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.P.; Yuen, K.H.; Wong, J.W. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J. Pharm. Pharmacol. 2001, 53, 67–71. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Khan, D.A.; Silswal, N.; Saleem, S.; Qureshi, N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.; Khan, D.; Saleem, S.; Silswal, N.; Trias, A.; Tan, B. Pharmacokinetics and bioavailability of annatto δ-tocotrienol in healthy fed subjects. J. Clin. Exp. Cardiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Satyamitra, M.; Ney, P.; Graves, J., 3rd; Mullaney, C.; Srinivasan, V. Mechanism of radioprotection by delta-tocotrienol: Pharmacokinetics, pharmacodynamics and modulation of signalling pathways. Br. J. Radiol. 2012, 85, e1093–e1103. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Lee, M.-J.; Zhao, Y.; Yang, C.S. Metabolism of tocotrienols in animals and synergistic inhibitory actions of tocotrienols with atorvastatin in cancer cells. Genes Nutr. 2012, 7, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, Y.; Tsuzuki, T.; Nakagawa, K.; Miyazawa, T. Distribution of tocotrienols in rats fed a rice bran tocotrienol concentrate. Biosci. Biotechnol. Biochem. 2007, 71, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Abe, C.; Nomura, S.; Ichikawa, T.; Ikeda, S. Tissue distribution of alpha- and gamma-tocotrienol and gamma-tocopherol in rats and interference with their accumulation by alpha-tocopherol. Lipids 2012, 47, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Patel, V.; Rink, C.; Roy, S.; Sen, C.K. Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic. Biol. Med. 2005, 39, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.; Wang, T.; Dolde, D.; Xin, H. Tocopherol and annatto tocotrienols distribution in laying-hen body. Poult. Sci. 2015, 94, 2421–2433. [Google Scholar] [CrossRef]
- Birringer, M.; Pfluger, P.; Kluth, D.; Landes, N.; Brigelius-Flohe, R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002, 132, 3113–3118. [Google Scholar] [CrossRef]
- Pein, H.; Ville, A.; Pace, S.; Temml, V.; Garscha, U.; Raasch, M.; Alsabil, K.; Viault, G.; Dinh, C.P.; Guilet, D.; et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat. Commun. 2018, 9, 3834. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.P.; Yuen, K.H. Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations. Int. J. Pharm. 2004, 281, 67–78. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-F.; Chang, Y.-H.; Chien, S.-C.; Lin, Y.-H.; Yeh, H.-Y. Epidemiology of Dyslipidemia in the Asia Pacific Region. Int. J. Gerontol. 2018, 12, 2–6. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory (GHO) Data: Raised Cholesterol. 2008. Available online: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 10 May 2019).
- Jamal, R.; Syed Zakaria, S.Z.; Kamaruddin, M.A.; Abd Jalal, N.; Ismail, N.; Mohd Kamil, N.; Abdullah, N.; Baharudin, N.; Hussin, N.H.; Othman, H.; et al. Cohort Profile: The Malaysian Cohort (TMC) project: A prospective study of non-communicable diseases in a multi-ethnic population. Int. J. Epidemiol. 2015, 44, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.; et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Adam, S.; Mohammad, J.B.; Ho, J.H.; Schofield, J.D.; Kwok, S.; Siahmansur, T.; Liu, Y.; Syed, A.A.; Dhage, S.S.; et al. Hypercholesterolaemia—Practical information for non-specialists. Arch. Med. Sci. AMS 2018, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.-J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar]
- Cimminiello, C.; Zambon, A.; Polo Friz, H. Hypercholesterolemia and cardiovascular risk: Advantages and limitations of current treatment options. Giornale Italiano di Cardiologia 2016, 17, 6s–13s. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 97–130. [Google Scholar] [CrossRef]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar]
- Burdeos, G.C.; Nakagawa, K.; Watanabe, A.; Kimura, F.; Miyazawa, T. gamma-Tocotrienol attenuates triglyceride through effect on lipogenic gene expressions in mouse hepatocellular carcinoma Hepa 1-6. J. Nutr. Sci. Vitaminol. 2013, 59, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Burdeos, G.C.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol attenuates triglyceride accumulation in HepG2 cells and F344 rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef]
- Kumarappan, C. Polyphenolic extract of Ichnocarpus frutescens modifies hyperlipidemia status in diabetic rats. J. Cell Mol. Biol. 2007, 6, 175–187. [Google Scholar]
- Nakamura, H.; Furukawa, F.; Nishikawa, A.; Miyauchi, M.; Son, H.Y.; Imazawa, T.; Hirose, M. Oral toxicity of a tocotrienol preparation in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2001, 39, 799–805. [Google Scholar] [CrossRef]
- Song, B.L.; DeBose-Boyd, R.A. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J. Biol. Chem. 2006, 281, 25054–25061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman Khan, M.; Akhtar, S.; Al-Sagair, O.A.; Arif, J.M. Protective effect of dietary tocotrienols against infection and inflammation-induced hyperlipidemia: An in vivo and in silico study. Phytother. Res. PTR 2011, 25, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Theriault, A.; Wang, Q.; Gapor, A.; Adeli, K. Effects of gamma-tocotrienol on ApoB synthesis, degradation, and secretion in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Zaiden, N.; Yap, W.N.; Ong, S.; Xu, C.H.; Teo, V.H.; Chang, C.P.; Zhang, X.W.; Nesaretnam, K.; Shiba, S.; Yap, Y.L. Gamma delta tocotrienols reduce hepatic triglyceride synthesis and VLDL secretion. J. Atheroscler. Thromb. 2010, 17, 1019–1032. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A.; Peterson, D.M.; Elson, C.E. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J. Nutr. 1996, 126, 389–394. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Peterson, D.M. The combined effects of novel tocotrienols and lovastatin on lipid metabolism in chickens. Atherosclerosis 2001, 156, 39–47. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Reis, J.C.; Qureshi, N.; Papasian, C.J.; Morrison, D.C.; Schaefer, D.M. delta-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis. 2011, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Qureshi, N.; Hasler-Rapacz, J.O.; Weber, F.E.; Chaudhary, V.; Crenshaw, T.D.; Gapor, A.; Ong, A.S.; Chong, Y.H.; Peterson, D. Dietary tocotrienols reduce concentrations of plasma cholesterol, apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inherited hyperlipidemias. Am. J. Clin. Nutr. 1991, 53, 1042s–1046s. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Peterson, D.M.; Hasler-Rapacz, J.O.; Rapacz, J. Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J. Nutr. 2001, 131, 223–230. [Google Scholar] [CrossRef]
- Yu, S.G.; Thomas, A.M.; Gapor, A.; Tan, B.; Qureshi, N.; Qureshi, A.A. Dose-response impact of various tocotrienols on serum lipid parameters in 5-week-old female chickens. Lipids 2006, 41, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Watkins, T.; Lenz, P.; Gapor, A.; Struck, M.; Tomeo, A.; Bierenbaum, M. gamma-Tocotrienol as a hypocholesterolemic and antioxidant agent in rats fed atherogenic diets. Lipids 1993, 28, 1113–1118. [Google Scholar] [CrossRef]
- Kaku, S.; Yunoki, S.; Mori, M.; Ohkura, K.; Nonaka, M.; Sugano, M.; Yamada, K. Effect of dietary antioxidants on serum lipid contents and immunoglobulin productivity of lymphocytes in Sprague-Dawley rats. Biosci. Biotechnol. Biochem. 1999, 63, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2005, 43, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Ton, S.H.; Tan, J.B.L.; Abdul Kadir, K. The Ameliorative Effects of a Tocotrienol-Rich Fraction on the AGE-RAGE Axis and Hypertension in High-Fat-Diet-Fed Rats with Metabolic Syndrome. Nutrients 2017, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Budin, S.B.; Othman, F.; Louis, S.R.; Bakar, M.A.; Das, S.; Mohamed, J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics 2009, 64, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Minhajuddin, M.; Beg, Z.H. Suppression of 7,12-dimethylbenz[alpha]anthracene-induced carcinogenesis and hypercholesterolaemia in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2003, 12, 447–453. [Google Scholar] [CrossRef]
- Khor, H.T.; Chieng, D.Y.; Ong, K.K. Tocotrienols inhibit liver HMG CoA reductase activity in the guinea pig. Nutr. Res. 1995, 15, 537–544. [Google Scholar] [CrossRef]
- Khor, H.T.; Chieng, D.Y. Lipidaemic effects of tocotrienols, tocopherols and squalene: Studies in the hamster. Asia Pac. J. Clin. Nutr. 1997, 6, 36–40. [Google Scholar]
- Raederstorff, D.; Elste, V.; Aebischer, C.; Weber, P. Effect of either gamma-tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters. Ann. Nutr. Metab. 2002, 46, 17–23. [Google Scholar] [CrossRef]
- Khor, H.T.; Ng, T.T. Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int. J. Food Sci. Nutr. 2000, 51, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Khor, H.; Ng, T.; Rajendran, R. Dose-dependent cholesterolemic activity of tocotrienols. Malays. J. Nutr. 2002, 8, 157–166. [Google Scholar] [PubMed]
- Qureshi, A.A.; Qureshi, N.; Wright, J.J.; Shen, Z.; Kramer, G.; Gapor, A.; Chong, Y.H.; DeWitt, G.; Ong, A.; Peterson, D.M. Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (palmvitee). Am. J. Clin. Nutr. 1991, 53, 1021s–1026s. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Bradlow, B.A.; Brace, L.; Manganello, J.; Peterson, D.M.; Pearce, B.C.; Wright, J.J.; Gapor, A.; Elson, C.E. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids 1995, 30, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J. Nutr. Biochem. 2001, 12, 318–329. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef]
- Yuen, K.H.; Wong, J.W.; Lim, A.B.; Ng, B.H.; Choy, W.P. Effect of Mixed-Tocotrienols in Hypercholesterolemic Subjects. Funct. Foods Health Dis. 2011, 3, 106–117. [Google Scholar] [CrossRef]
- Ajuluchukwu, J.N.; Okubadejo, N.U.; Mabayoje, M.; Ojini, F.I.; Okwudiafor, R.N.; Mbakwem, A.C.; Fasanmade, O.A.; Oke, D.A. Comparative study of the effect of tocotrienols and -tocopherol on fasting serum lipid profiles in patients with mild hypercholesterolaemia: A preliminary report. Niger. Postgrad. Med. J. 2007, 14, 30–33. [Google Scholar]
- Heng, K.; Hejar, A.; Johnson, S.J.; Ooi, C.; Loh, S. Potential of Mixed Tocotrienol Supplementation to Reduce Cholesterol and Cytokines Level in Adults with Metabolic Syndrome. Malays. J. Nutr. 2015, 21, 231–243. [Google Scholar]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef]
- Daud, Z.A.; Tubie, B.; Sheyman, M.; Osia, R.; Adams, J.; Tubie, S.; Khosla, P. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc. Health Risk Manag. 2013, 9, 747–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.T.; Khor, H.T.; Low, W.H.; Ali, A.; Gapor, A. Effect of a palm-oil-vitamin E concentrate on the serum and lipoprotein lipids in humans. Am. J. Clin. Nutr. 1991, 53, 1027s–1030s. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Ibahim, J.; Makpol, S.; Abdul Hamid, N.A.; Abdul Latiff, A.; Zakaria, Z.; Mazlan, M.; Mohd Yusof, Y.A.; Abdul Karim, A.; Wan Ngah, W.Z. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr. Metab. 2011, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heng, E.C.; Karsani, S.A.; Abdul Rahman, M.; Abdul Hamid, N.A.; Hamid, Z.; Wan Ngah, W.Z. Supplementation with tocotrienol-rich fraction alters the plasma levels of Apolipoprotein A-I precursor, Apolipoprotein E precursor, and C-reactive protein precursor from young and old individuals. Eur. J. Nutr. 2013, 52, 1811–1820. [Google Scholar] [CrossRef]
- Tomeo, A.C.; Geller, M.; Watkins, T.R.; Gapor, A.; Bierenbaum, M.L. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids 1995, 30, 1179–1183. [Google Scholar] [CrossRef]
- Mensink, R.P.; van Houwelingen, A.C.; Kromhout, D.; Hornstra, G. A vitamin E concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid concentrations. Am. J. Clin. Nutr. 1999, 69, 213–219. [Google Scholar] [CrossRef] [Green Version]
- O’Byrne, D.; Grundy, S.; Packer, L.; Devaraj, S.; Baldenius, K.; Hoppe, P.P.; Kraemer, K.; Jialal, I.; Traber, M.G. Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic. Biol. Med. 2000, 29, 834–845. [Google Scholar] [CrossRef]
- Mustad, V.A.; Smith, C.A.; Ruey, P.P.; Edens, N.K.; DeMichele, S.J. Supplementation with 3 compositionally different tocotrienol supplements does not improve cardiovascular disease risk factors in men and women with hypercholesterolemia. Am. J. Clin. Nutr. 2002, 76, 1237–1243. [Google Scholar] [CrossRef]
- Rasool, A.H.; Yuen, K.H.; Yusoff, K.; Wong, A.R.; Rahman, A.R. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J. Nutr. Sci. Vitaminol. 2006, 52, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wei, F.F.; Wang, S.; Cheng, Y.B.; Wang, J.G. Cardiovascular risks associated with diastolic blood pressure and isolated diastolic hypertension. Curr. Hypertens. Rep. 2014, 16, 489. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 9 August 2019).
- Leong, X.F.; Salimon, J.; Mustafa, M.R.; Jaarin, K. Effect of repeatedly heated palm olein on blood pressure-regulating enzymes activity and lipid peroxidation in rats. Malays. J. Med Sci. Mjms 2012, 19, 20–29. [Google Scholar] [PubMed]
- Ng, C.Y.; Kamisah, Y.; Faizah, O.; Jaarin, K. The role of repeatedly heated soybean oil in the development of hypertension in rats: Association with vascular inflammation. Int. J. Exp. Pathol. 2012, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Leong, X.F.; Masbah, N.; Adam, S.K.; Kamisah, Y.; Jaarin, K. Heated vegetable oils and cardiovascular disease risk factors. Vasc. Pharmacol. 2014, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Q.; Ren, W.; Peng, R.; Liu, Y.H.; Li, Y. Clinical application of endothelial injury marker in hypertensive patients. J. Clin. Lab. Anal. 2018, 32, e22387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamisah, Y.; Ang, S.M.; Othman, F.; Nurul-Iman, B.S.; Qodriyah, H.M. Renoprotective effect of virgin coconut oil in heated palm oil diet-induced hypertensive rats. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2016, 41, 1033–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamisah, Y.; Zuhair, J.S.F.; Juliana, A.H.; Jaarin, K. Parkia speciosa empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-l-arginine methyl ester. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 96, 291–298. [Google Scholar] [CrossRef]
- Vergely, C.; Maupoil, V.; Clermont, G.; Bril, A.; Rochette, L. Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch. Biochem. Biophys. 2003, 420, 209–216. [Google Scholar] [CrossRef]
- Baszczuk, A.; Kopczynski, Z. Hyperhomocysteinemia in patients with cardiovascular disease. Postepy Higieny i Medycyny Doswiadczalnej 2014, 68, 579–589. [Google Scholar] [CrossRef]
- Norsidah, K.-Z.; Asmadi, A.Y.; Azizi, A.; Faizah, O.; Kamisah, Y. Palm Tocotrienol-Rich Fraction Improves Vascular Proatherosclerotic Changes in Hyperhomocysteinemic Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Norsidah, K.Z.; Asmadi, A.Y.; Azizi, A.; Faizah, O.; Kamisah, Y. Palm tocotrienol-rich fraction reduced plasma homocysteine and heart oxidative stress in rats fed with a high-methionine diet. J. Physiol. Biochem. 2013, 69, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Lazou, A.; Gorbe, A.; Giricz, Z.; Schulz, R.; Ferdinandy, P. Effect of hypercholesterolaemia on myocardial function, ischaemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2017, 174, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Das, S.; Wang, P.; Powell, S.R.; Das, D.K. Caveolin and proteasome in tocotrienol mediated myocardial protection. Cell Physiol. Biochem. 2008, 22, 287–294. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, S.; Lekli, I.; Gurusamy, N.; Bardhan, J.; Raychoudhury, U.; Chakravarty, R.; Banerji, S.; Knowlton, A.A.; Das, D.K. Tocotrienols confer resistance to ischemia in hypercholesterolemic hearts: Insight with genomics. Mol. Cell. Biochem. 2012, 360, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.; Adam, A.; Ngah, W.W.; Gapor, A.; Khalid, B.A.; Marzuki, A. Tocotrienol and tocopherol were protective against xanthine plus xanthine oxidase induced oxidative stress. Asia Pac. J. Pharmacol. 2000, 14, 111–116. [Google Scholar]
- Kamisah, Y.; Norsidah, K.Z.; Azizi, A.; Faizah, O.; Nonan, M.R.; Asmadi, A.Y. Palm tocotrienol-rich fraction inhibits methionine-induced cystathionine beta-synthase in rat liver. J. Physiol. Biochem. 2015, 71, 659–667. [Google Scholar] [CrossRef]
- Joseph, J.; Loscalzo, J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013, 5, 3235–3256. [Google Scholar] [CrossRef] [Green Version]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Morrow, J.D.; Shooter, L.A.; Holmes, S.; Heward, C.; Henson, D.A. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J. Nutr. Biochem. 2005, 16, 530–537. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Poudyal, H.; Ward, L.C.; Brown, L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients 2012, 4, 1527–1541. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory gamma- and delta-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef]
- Muharis, S.P.; Top, A.G.; Murugan, D.; Mustafa, M.R. Palm oil tocotrienol fractions restore endothelium dependent relaxation in aortic rings of streptozotocin-induced diabetic and spontaneously hypertensive rats. Nutr. Res. 2010, 30, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Newaz, M.A.; Yousefipour, Z.; Nawal, N.; Adeeb, N. Nitric oxide synthase activity in blood vessels of spontaneously hypertensive rats: Antioxidant protection by gamma-tocotrienol. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2003, 54, 319–327. [Google Scholar]
- Mahdy, Z.A.; Siraj, H.H.; Khaza’ai, H.; Mutalib, M.S.; Azwar, M.H.; Wahab, M.A.; Dali, A.Z.; Jaafar, R.; Ismail, N.A.; Jamil, M.A.; et al. Does palm oil vitamin E reduce the risk of pregnancy induced hypertension? Acta Med. 2013, 56, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, A.H.; Rahman, A.R.; Yuen, K.H.; Wong, A.R. Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch. Pharmacal. Res. 2008, 31, 1212–1217. [Google Scholar] [CrossRef]
- Catalina-Romero, C.; Ruilope, L.M.; Sanchez-Chaparro, M.A.; Valdivielso, P.; Cabrera-Sierra, M.; Fernandez-Labandera, C.; Ruiz-Moraga, M.; Gonzalez-Quintela, A.; Calvo-Bonacho, E. Factors influencing return-to-work after cerebrovascular disease: The importance of previous cardiovascular risk. Eur. J. Prev. Cardiol. 2015, 22, 1220–1227. [Google Scholar] [CrossRef]
- Rink, C.; Christoforidis, G.; Khanna, S.; Peterson, L.; Patel, Y.; Khanna, S.; Abduljalil, A.; Irfanoglu, O.; Machiraju, R.; Bergdall, V.K.; et al. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011, 31, 2218–2230. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Rink, C.; Ghoorkhanian, R.; Gnyawali, S.; Heigel, M.; Wijesinghe, D.S.; Chalfant, C.E.; Chan, Y.C.; Banerjee, J.; Huang, Y.; et al. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Park, H.A.; Kubicki, N.; Gnyawali, S.; Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Natural vitamin E alpha-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 2011, 42, 2308–2314. [Google Scholar] [CrossRef] [Green Version]
- Mishima, K.; Tanaka, T.; Pu, F.; Egashira, N.; Iwasaki, K.; Hidaka, R.; Matsunaga, K.; Takata, J.; Karube, Y.; Fujiwara, M. Vitamin E isoforms alpha-tocotrienol and gamma-tocopherol prevent cerebral infarction in mice. Neurosci. Lett. 2003, 337, 56–60. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Slivka, A.; Craft, T.K.; Chaki, S.; Rink, C.; Notestine, M.A.; DeVries, A.C.; Parinandi, N.L.; Sen, C.K. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 2005, 36, 2258–2264. [Google Scholar] [CrossRef]
- Kole, A.J.; Swahari, V.; Hammond, S.M.; Deshmukh, M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011, 25, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.A.; Khanna, S.; Rink, C.; Gnyawali, S.; Roy, S.; Sen, C.K. Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell Death Differ. 2009, 16, 1167–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Shang, J.; Ohta, Y.; Yan, H.; Liu, X.; Li, X.; Morihara, R.; Nakano, Y.; Fukui, Y.; Shi, X.; et al. Neuroprotective Effects of Tocovid Pretreatment in a Mouse Stroke Model. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2018, 27, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Yan, H.; Jiao, Y.; Ohta, Y.; Liu, X.; Li, X.; Morihara, R.; Nakano, Y.; Fukui, Y.; Shi, X.; et al. Therapeutic Effects of Pretreatment with Tocovid on Oxidative Stress in Postischemic Mice Brain. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2018, 27, 2096–2105. [Google Scholar] [CrossRef]
- Gopalan, Y.; Shuaib, I.L.; Magosso, E.; Ansari, M.A.; Abu Bakar, M.R.; Wong, J.W.; Khan, N.A.; Liong, W.C.; Sundram, K.; Ng, B.H.; et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke 2014, 45, 1422–1428. [Google Scholar] [CrossRef] [Green Version]
- Oden, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Duque, G.; Rivas, D.; Li, W.; Li, A.; Henderson, J.E.; Ferland, G.; Gaudreau, P. Age-related bone loss in the LOU/c rat model of healthy ageing. Exp. Gerontol. 2009, 44, 183–189. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Zulkepli, M.A.A.C.; Theseira, K.M.; Zulkifli, N.; Shahrom, N.Q.; Ridzuan, N.A.M.; Jamil, N.A.; Soelaiman, I.-N.; Chin, K.-Y. Establishing an animal model of secondary osteoporosis by using a gonadotropin-releasing hormone agonist. Int. J. Med Sci. 2018, 15, 300. [Google Scholar] [CrossRef] [Green Version]
- Broulik, P.D.; Vondrova, J.; Ruzicka, P.; Sedlacek, R.; Zima, T. The effect of chronic alcohol administration on bone mineral content and bone strength in male rats. Physiol. Res. 2010, 59, 599–604. [Google Scholar] [PubMed]
- Broulik, P.D.; Rosenkrancova, J.; Ruzicka, P.; Sedlacek, R.; Kurcova, I. The effect of chronic nicotine administration on bone mineral content and bone strength in normal and castrated male rats. Horm. Metab. Res. 2007, 39, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lu, H.; Liu, P. Association between essential hypertension and bone mineral density: A systematic review and meta-analysis. Oncotarget 2017, 8, 68916. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.P.; Singh, A.K.; Joharapurkar, A.A.; Yadav, M.; Shree, S.; Kumar, H.; Gurjar, A.; Mishra, J.S.; Tiwari, M.C.; Nagar, G.K.; et al. Pathophysiological Mechanism of Bone Loss in Type 2 Diabetes Involves Inverse Regulation of Osteoblast Function by PGC-1alpha and Skeletal Muscle Atrogenes: AdipoR1 as a Potential Target for Reversing Diabetes-Induced Osteopenia. Diabetes 2015, 64, 2609–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirih, F.; Lu, J.; Ye, F.; Bezouglaia, O.; Atti, E.; Ascenzi, M.G.; Tetradis, S.; Demer, L.; Aghaloo, T.; Tintut, Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J. Bone Miner. Res. 2012, 27, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS ONE 2018, 13, e0192416. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Jamil, N.A.; Ima-Nirwana, S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed. Pharmacother. 2018, 98, 191–200. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Norazlina, M.; Chua, C.W.; Ima-Nirwana, S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med. J. Malays. 2004, 59, 623–630. [Google Scholar]
- Norazlina, M.; Ima-Nirwana, S.; Abul Gapor, M.T.; Abdul Kadir Khalid, B. Tocotrienols are needed for normal bone calcification in growing female rats. Asia Pac. J. Clin. Nutr. 2002, 11, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Norazlina, M.; Ima-Nirwana, S.; Gapor, M.T.; Khalid, B.A. Palm vitamin E is comparable to alpha-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp. Clin. Endocrinol. Diabetes 2000, 108, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Mohd Ali, H.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, N.; Luke, D.A.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid.-Based Complement. Altern. Med. 2012, 2012, 161527. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Abdul-Majeed, S.; Mohamed, N.; Ima-Nirwana, S. The Effects of Tocotrienol and Lovastatin Co-Supplementation on Bone Dynamic Histomorphometry and Bone Morphogenetic Protein-2 Expression in Rats with Estrogen Deficiency. Nutrients 2017, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Ding, Y.; Peng, Y.; Wu, Y.; Fan, J.; Li, W.; Yang, R.; Yang, M.; Fu, Q. gamma-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 2014, 67, 200–207. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid.-Based Complement. Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015, 125, 42–48. [Google Scholar] [CrossRef]
- Aktifanus, A.T.; Shuid, A.N.; Rashid, N.H.; Ling, T.H.; Loong, C.Y.; Saat, N.M.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J. Anim. Vet. Adv. 2012, 7, 225–234. [Google Scholar]
- Mohamad, S.; Shuid, A.N.; Mokhtar, S.A.; Abdullah, S.; Soelaiman, I.N. Tocotrienol supplementation improves late-phase fracture healing compared to alpha-tocopherol in a rat model of postmenopausal osteoporosis: A biomechanical evaluation. Evid.-Based Complement. Altern. Med. 2012, 2012, 372878. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.; Khamis, M.F.; Mod Yunoh, M.F.; Abdullah, S.; Mohamed, N.; Shuid, A.N. Targeted delivery of lovastatin and tocotrienol to fracture site promotes fracture healing in osteoporosis model: Micro-computed tomography and biomechanical evaluation. PLoS ONE 2014, 9, e115595. [Google Scholar] [CrossRef] [Green Version]
- Mehat, M.Z.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J. Bone Miner. Metab. 2010, 28, 503–509. [Google Scholar] [CrossRef]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Ahmad Nazrun, S.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague-dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Khalid, B.A.K.; Luke, D.A.; Ima Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Effects of Vitamin E from Elaeis guineensis (Oil Palm) in a Rat Model of Bone Loss Due to Metabolic Syndrome. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [Green Version]
- Tennant, K.G.; Leonard, S.W.; Wong, C.P.; Iwaniec, U.T.; Turner, R.T.; Traber, M.G. High-Dietary Alpha-Tocopherol or Mixed Tocotrienols Have No Effect on Bone Mass, Density, or Turnover in Male Rats During Skeletal Maturation. J. Med. Food 2017, 20, 700–708. [Google Scholar] [CrossRef]
- Chin, K.Y.; Abdul-Majeed, S.; Fozi, N.F.; Ima-Nirwana, S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients 2014, 6, 4974–4983. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Kiftiah, A.; Zainal, A.; Norazlina, M.; Gapor, M.; Khalid, B. Palm vitamin E prevents osteoporosis in orchidectomized growing male rats. Nat. Prod. Sci. 2000, 6, 155–160. [Google Scholar]
- Ima-Nirwana, S.; Fakhrurazi, H. Palm vitamin E protects bone against dexamethasone-induced osteoporosis in male rats. Med. J. Malays. 2002, 57, 136–144. [Google Scholar]
- Ima-Nirwana, S.; Suhaniza, S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Maizatul-Neza, J.; Azarina, A.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Effects of vitamin E on receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) in rats treated with nicotine. Med. J. Malays. 2010, 65, 14–17. [Google Scholar]
- Shuid, A.N.; Mehat, Z.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Vitamin E exhibits bone anabolic actions in normal male rats. J. Bone Miner. Metab. 2010, 28, 149–156. [Google Scholar] [CrossRef]
- Chin, K.Y.; Gengatharan, D.; Mohd Nasru, F.S.; Khairussam, R.A.; Ern, S.L.; Aminuddin, S.A.; Ima-Nirwana, S. The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients 2016, 8, 808. [Google Scholar] [CrossRef] [Green Version]
- Nazrun, A.S.; Khairunnur, A.; Norliza, M.; Norazlina, M.; Ima Nirwana, S. Effects of Palm Tocotrienols on Oxidative Stress and Bone Strength in Ovariectomised Rats. Med. Health 2008, 3, 247–255. [Google Scholar]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm tocotrienol exerted better antioxidant activities in bone than alpha-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef]
- Muhammad, N.; Luke, D.A.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Tocotrienol supplementation in postmenopausal osteoporosis: Evidence from a laboratory study. Clinics 2013, 68, 1338–1343. [Google Scholar] [CrossRef]
- Norazlina, M.; Lee, P.L.; Lukman, H.I.; Nazrun, A.S.; Ima-Nirwana, S. Effects of vitamin E supplementation on bone metabolism in nicotine-treated rats. Singap. Med. J. 2007, 48, 195–199. [Google Scholar]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Norazlina, M.; Hermizi, H.; Faizah, O.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch Med. Sci. 2010, 6, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Abukhadir, S.S.A.; Mohamed, N.; Makpol, S.; Muhammad, N. Effects of palm vitamin E on bone-formation-related gene expression in nicotine-treated rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 656025. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Mohamed, N.; Soelaiman, I.N.; Shuid, A.N. The Effects of Targeted Deliveries of Lovastatin and Tocotrienol on Ossification-Related Gene Expressions in Fracture Healing in an Osteoporosis Rat Model. Int. J. Environ. Res. Public Health 2015, 12, 12958–12976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan Hasan, W.N.; Abd Ghafar, N.; Chin, K.Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: A temporal sequential study. Drug Des. Dev. 2018, 12, 1715–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Tudawe, D.; Chakravarthi, S.; Chiew, G.S.; Haleagrahara, N. Effect of gamma-tocotrienol in counteracting oxidative stress and joint damage in collagen-induced arthritis in rats. Exp. Ther. Med. 2014, 7, 1408–1414. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Swaminathan, M.; Chakravarthi, S.; Radhakrishnan, A. Therapeutic efficacy of vitamin E delta-tocotrienol in collagen-induced rat model of arthritis. Biomed. Res. Int. 2014, 2014, 539540. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Wong, S.K.; Japar Sidik, F.Z.; Abdul Hamid, J.; Abas, N.H.; Mohd Ramli, E.S.; Afian Mokhtar, S.; Rajalingham, S.; Ima Nirwana, S. The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Zainal, Z.; Rahim, A.A.; Radhakrishnan, A.K.; Chang, S.K.; Khaza’ai, H. Investigation of the curative effects of palm vitamin E tocotrienols on autoimmune arthritis disease in vivo. Sci. Rep. 2019, 9, 16793. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef]
- Sehl, M.E.; Yates, F.E. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J. Gerontol. Biol. Sci. Med. Sci. 2001, 56, B198–B208. [Google Scholar] [CrossRef] [Green Version]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.E.; Montine, T.J.; Motley, A.K.; Li, X.; May, J.M.; Burk, R.F. Combined deficiency of vitamins E and C causes paralysis and death in guinea pigs. Am. J. Clin. Nutr. 2003, 77, 1484–1488. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.E.; Motley, A.K.; May, J.M.; Burk, R.F. Combined selenium and vitamin C deficiency causes cell death in guinea pig skeletal muscle. Nutr. Res. 2009, 29, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.R.; Traber, M.G.; Kayden, H.J.; Cox, N.R.; Toivio-Kinnucan, M.; Wright, J.C.; Braund, K.G.; Whitley, R.D.; Gilger, B.C.; Steiss, J.E. Concomitant brainstem axonal dystrophy and necrotizing myopathy in vitamin E-deficient rats. J. Neurol. Sci. 1994, 123, 64–73. [Google Scholar] [CrossRef]
- Thomas, P.K.; Cooper, J.M.; King, R.H.; Workman, J.M.; Schapira, A.H.; Goss-Sampson, M.A.; Muller, D.P. Myopathy in vitamin E deficient rats: Muscle fibre necrosis associated with disturbances of mitochondrial function. J. Anat. 1993, 183, 451–461. [Google Scholar] [PubMed]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Victor, P.; Gutarra, S.; Garcia-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardi, M.; Ballestar, E.; Gonzalez, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yakabe, M.; Akishita, M. Age-related sarcopenia and its pathophysiological bases. Inflamm. Regen. 2016, 36, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lin, S.; Gao, T.; Zhong, F.; Cai, J.; Sun, Y.; Ma, A. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kefaloyianni, E.; Gaitanaki, C.; Beis, I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal 2006, 18, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Gallagher, P.; Harber, M.; Hollon, C.; Trappe, S. Mitogen-activated protein kinase (MAPK) pathway activation: Effects of age and acute exercise on human skeletal muscle. J. Physiol. 2003, 547, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Wan Zurinah, W.N.; Mouly, V.; Norwahidah, A.K. Tocotrienol-Rich Fraction (TRF) Treatment Promotes Proliferation Capacity of Stress-Induced Premature Senescence Myoblasts and Modulates the Renewal of Satellite Cells: Microarray Analysis. Oxid. Med. Cell Longev. 2019, 2019, 9141343. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Ngah, W.Z.; Mouly, V.; Abdul Karim, N. Reversal of myoblast aging by tocotrienol rich fraction posttreatment. Oxid. Med. Cell Longev. 2013, 2013, 978101. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.C.; Razak, A.M.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Abdul Karim, N.; Makpol, S. The Tocotrienol-Rich Fraction Is Superior to Tocopherol in Promoting Myogenic Differentiation in the Prevention of Replicative Senescence of Myoblasts. PLoS ONE 2016, 11, e0149265. [Google Scholar] [CrossRef]
- Ong, F.B.; Wan Ngah, W.Z.; Shamaan, N.A.; Md Top, A.G.; Marzuki, A.; Khalid, A.K. Glutathione S-transferase and gamma-glutamyl transpeptidase activities in cultured rat hepatocytes treated with tocotrienol and tocopherol. Comp. Biochem. Physiol. C 1993, 106, 237–240. [Google Scholar] [CrossRef]
- Osakada, F.; Hashino, A.; Kume, T.; Katsuki, H.; Kaneko, S.; Akaike, A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 2004, 47, 904–915. [Google Scholar] [CrossRef]
- Palozza, P.; Verdecchia, S.; Avanzi, L.; Vertuani, S.; Serini, S.; Iannone, A.; Manfredini, S. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol. Cell. Biochem. 2006, 287, 21–32. [Google Scholar] [CrossRef]
- Chin, S.F.; Hamid, N.A.; Latiff, A.A.; Zakaria, Z.; Mazlan, M.; Yusof, Y.A.; Karim, A.A.; Ibahim, J.; Hamid, Z.; Ngah, W.Z. Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation. Nutrition 2008, 24, 1–10. [Google Scholar] [CrossRef]
- Momma, H.; Niu, K.; Kobayashi, Y.; Guan, L.; Sato, M.; Guo, H.; Chujo, M.; Otomo, A.; Yufei, C.; Tadaura, H.; et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur. J. Appl. Physiol. 2011, 111, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Magasi, S. Identification of dynapenia in older adults through the use of grip strength t-scores. Muscle Nerve 2015, 51, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New perspectives on vitamin E: Gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef]
- Mohd, N.F.; Ibrahim, I.; Kamisah, Y.; Mohd, N.I. Palm vitamin E reduces catecholamines, xanthine oxidase activity and gastric lesions in rats exposed to water-immersion restraint stress. BMC Gastroenterol. 2012, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Fesharaki, M.; Nasimi, A.; Mokhtari, S.; Mokhtari, R.; Moradian, R.; Amirpoor, N. Reactive oxygen metabolites and anti-oxidative defenses in aspirin-induced gastric damage in rats: Gastroprotection by Vitamin E. Pathophysiology 2006, 13, 237–243. [Google Scholar] [CrossRef]
- Cuevas, V.M.; Calzado, Y.R.; Guerra, Y.P.; Yera, A.O.; Despaigne, S.J.; Ferreiro, R.M.; Quintana, D.C. Effects of grape seed extract, vitamin C, and vitamin E on ethanol-and aspirin-induced ulcers. Adv. Pharmacol. Sci. 2011, 2011, 740687. [Google Scholar] [CrossRef]
- Azlina, M.F.; Nafeeza, M.I.; Khalid, B.A.K. A comparison between tocopherol and tocotrienol effects on gastric parameters in rats exposed to stress. Asia Pac. J. Clin. Nutr. 2005, 14, 4. [Google Scholar]
- Bardhan, J.; Chakraborty, R.; Raychaudhuri, U. The 21st century form of vitamin E-Tocotrienol. Curr. Pharm. Des. 2011, 17, 2196–2205. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.A.A.; Kamisah, Y.; Nafeeza, M.I.; Azlina, M.F.N. The effects of palm vitamin E on stress hormone levels and gastric lesions in stress-induced rats. Arch. Med. Sci. AMS 2012, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Moreland, M.; Ames, B.N.; Yin, X. A combination of aspirin and γ-tocopherol is superior to that of aspirin and α-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J. Nutr. Biochem. 2009, 20, 894–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Lee, J.H.; Lee, S.S. Long-term stress and Helicobacter pylori infection independently induce gastric mucosal lesions in C57BL/6 mice. Scand. J. Gastroenterol. 2002, 37, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kojima, R.; Ito, M. Influence of aging on gastric ulcer healing activities of the antioxidants α-tocopherol and probucol. Eur. J. Pharmacol. 2008, 601, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Laloo, D.; Prasad, S.K.; Krishnamurthy, S.; Hemalatha, S. Gastroprotective activity of ethanolic root extract of Potentilla fulgens Wall. ex Hook. J. Ethnopharmacol. 2013, 146, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Harty, R.F. Stress-induced ulcer bleeding in critically ill patients. Gastroenterol. Clin. 2009, 38, 245–265. [Google Scholar] [CrossRef]
- Konturek, S.J.; Brzozowski, T.; Konturek, P.C.; Zwirska-Korczala, K.; Reiter, R.J. Day/night differences in stress-induced gastric lesions in rats with an intact pineal gland or after pinealectomy. J. Pineal Res. 2008, 44, 408–415. [Google Scholar] [CrossRef]
- Azlina, N.; Fahami, M.; Kamisah, Y.; Chua, K.H.; Qodriyah, H.M.S. Tocotrienol attenuates stress-induced gastric lesions via activation of prostaglandin and upregulation of COX-1 mRNA. Evid.-Based Complement. Altern. Med. 2013, 2013, 804796. [Google Scholar] [CrossRef] [Green Version]
- Kwiecien, S.; Brzozowski, T.; Konturek, S. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar]
- Kamisah, Y.; Ibrahim, A.; Nafeeza, M.; Nur-Azlina, M. Palm tocotrienol-rich fraction supplementation suppressed stress-induced gastric oxidative stress in rats. J. Appl. Pharm. Sci. 2011, 1, 118. [Google Scholar]
- Fahami, N.A.M.; Tajudin, M. Effects of Tocotrienol and Tocopherol Supplementation on Liver Oxidative Status and Antioxidant Enzymes Activity in Stress-Induced Rats. Sains Malays. 2011, 40, 481–487. [Google Scholar]
- Clària, J. Cyclooxygenase-2 biology. Curr. Pharm. Des. 2003, 9, 2177–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buharalioğlu, C.K.; Akar, F. The reactivity of serotonin, acetylcholine and kcl-induced contractions to relaxant agents in the rat gastric fundus. Pharmacol. Res. 2002, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bregonzio, C.; Armando, I.; Ando, H.; Jezova, M.; Baiardi, G.; Saavedra, J.M. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G414–G423. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Hatazawa, R.; Takahira, Y.; Izumi, N.; Filaretova, L.; Takeuchi, K. Preconditioning stress prevents cold restraint stress-induced gastric lesions in rats: Roles of COX-1, COX-2, and PLA 2. Dig. Dis. Sci. 2007, 52, 478–487. [Google Scholar] [CrossRef]
- Rodzian, M.N.S.; Ibrahim, I.A.A.; Fahami, N.A.M.; Ismail, N.M. Pure tocotrienol concentrate protected rat gastric mucosa from acute stress-induced injury by a non-antioxidant mechanism. Pol. J. Pathol. 2013, 64, 52–58. [Google Scholar] [CrossRef]
- Azlina, M.F.N.; Kamisah, Y.; Chua, K.H.; Ibrahim, I.A.A.; Qodriyah, H.M.S. Preventive effects of tocotrienol on stress-induced gastric mucosal lesions and its relation to oxidative and inflammatory biomarkers. PLoS ONE 2015, 10, e0139348. [Google Scholar] [CrossRef] [Green Version]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Lanas, A. Role of nitric oxide in the gastrointestinal tract. Arthritis Res. Ther. 2008, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Konturek, P.C.; Brzozowski, T.; Kania, J.; Konturek, S.J.; Kwiecien, S.; Pajdo, R.; Hahn, E.G. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rat. Eur. J. Pharm. 2003, 472, 213–220. [Google Scholar] [CrossRef]
- Antonisamy, P.; Kannan, P.; Aravinthan, A.; Duraipandiyan, V.; Valan Arasu, M.; Ignacimuthu, S.; Abdullah Al-Dhabi, N.; Kim, J.-H. Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: Investigation of potential mechanisms of action. Sci. World J. 2014, 2014, 616432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tétreault, M.P.; Chailler, P.; Beaulieu, J.F.; Rivard, N.; Ménard, D. Epidermal growth factor receptor-dependent PI3K-activation promotes restitution of wounded human gastric epithelial monolayers. J. Cell. Physiol. 2008, 214, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, D.; Yin, H. Effect of panax quinquefolius saponin on angiogenesis and expressions of VEGF and bFGF in myocardium of rats with acute myocardial infarction. Chin. J. Integr. Tradit. West. Med. 2007, 27, 331–334. [Google Scholar]
- Hull, M.A.; Hewett, P.W.; Brough, J.L.; Hawkey, C.J. Isolation and culture of human gastric endothelial cells. Gastroenterology 1996, 111, 1230–1240. [Google Scholar] [CrossRef]
- Azlina, M.F.N.; Qodriyah, H.M.S.; Chua, K.H.; Kamisah, Y. Comparison between tocotrienol and omeprazole on gastric growth factors in stress-exposed rats. World J. Gastroenterol. 2017, 23, 5887. [Google Scholar] [CrossRef]
- Nafeeza, M.I.; Kang, T.T. Synergistic effects of tocopherol, tocotrienol, and ubiquinone in indomethacin-induced experimental gastric lesions. Int. J. Vitam. Nutr. Res. 2005, 75, 149–155. [Google Scholar] [CrossRef]
- Nafeeza, M.I.; Fauzee, A.; Kamsiah, J.; Gapor, M. Comparative effects of a tocotrienol-rich fraction and tocopherol in aspirin-induced gastric lesions in rats. Asia Pac. J. Clin. Nutr. 2002, 11, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.Y.; Yeo, M.; Han, S.U.; Cho, Y.K.; Kim, Y.B.; Chung, M.H.; Kim, Y.S.; Cho, S.W.; Hahm, K.B. Synergism of Helicobacter pylori infection and stress on the augmentation of gastric mucosal damage and its prevention with alpha-tocopherol. Free Radic. Biol. Med. 2005, 38, 1447–1457. [Google Scholar] [CrossRef]
- Jaarin, K.; Gapor, M.T.; Nafeeza, M.I.; Fauzee, A.M. Effect of various doses of palm vitamin E and TF on aspirin-induced gastric lesions in rats. Int. J. Exp. Pathol. 2002, 83, 295–302. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Halici, Z.; Cakir, A.; Halici, M.; Aygun, H.; Suleyman, H.; Cadirci, E.; Atalay, F. Beneficial effects of vegetable oils (corn, olive and sunflower oils) and α-tocopherol on anti-inflammatory and gastrointestinal profiles of indomethacin in rats. Eur. J. Pharmacol. 2008, 591, 300–306. [Google Scholar] [CrossRef]
- Saad, Q.H.M.; Kassiml, N.M.; Top, G.M.; Ismail, N.M. Tocotrienol-rich Fraction and its Effects on Parameters Affecting Gastric Mucosal Integrity after a Single Exposure to Indomethacin. Pak. J. Nutr. 2002, 1, 89–92. [Google Scholar]
- Ohta, Y.; Kobayashi, T.; Imai, Y.; Inui, K.; Yoshino, J.; Nakazawa, S. Effect of Oral Vitamin E Administration on Acute Gastric Mucosal Lesion Progression in Rats Treated with Compound 48/80, a Mast Cell Degranulator. Biol. Pharm. Bull. 2006, 29, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Jaarin, K.; Renuvathani, M.; Nafeeza, M.I.; Gapor, M.T. Effect of palm vitamin E on the healing of ethanol-induced gastric injury in rats. Int. J. Food Sci. Nutr. 2000, 51, s31–s41. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. WJG 2012, 18, 2147. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, J.; Song, C.-J.; Bian, J.-S.; Sparatore, A.; Del Soldato, P.; Wang, X.-Y.; Yan, C.-D. H (2) S-releasing aspirin protects against aspirin-induced gastric injury via reducing oxidative stress. PLoS ONE 2012, 7, e46301. [Google Scholar] [CrossRef]
- Jaarin, K.; Renuvathani, M.; Nafeeza, M.I.; Gapor, M.T. Comparative effect of palm vitamin E and ranitidine on the healing of ethanol-induced gastric lesions in rats. Int. J. Exp. Pathol. 1999, 80, 259–263. [Google Scholar] [CrossRef]
- Ismail, N.M.; Jaarin, K.; Ahmad, A.; Marzuki, A.; Ng, W.K.; Gapor, M.T. Palm vitamin E and the healing of ethanol-induced gastric lesions. Asia Pac. J. Clin. Nutr. 1999, 8, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.A.A.; Kamisah, Y.; Nafeeza, M.I.; Nur Azlina, M. Modulation of gastric motility and gastric lesion formation in stressed rats given enteral supplementation of palm vitamin E and a-tocopherol. Int. Med. J. 2011, 18, 47–52. [Google Scholar]
- Sen, C.K.; Rink, C.; Khanna, S. Palm oil-derived natural vitamin E alpha-tocotrienol in brain health and disease. J. Am. Coll. Nutr. 2010, 29, 314S–323S. [Google Scholar] [CrossRef]
- Schaffer, S.; Muller, W.E.; Eckert, G.P. Tocotrienols: Constitutional effects in aging and disease. J. Nutr. 2005, 135, 151–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Ghebremedhin, E.; Rub, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 120–131. [Google Scholar] [CrossRef]
- Nakaso, K.; Tajima, N.; Horikoshi, Y.; Nakasone, M.; Hanaki, T.; Kamizaki, K.; Matsura, T. The estrogen receptor beta-PI3K/Akt pathway mediates the cytoprotective effects of tocotrienol in a cellular Parkinson’s disease model. Biochim. Biophys. Acta 2014, 1842, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Nakaso, K.; Horikoshi, Y.; Takahashi, T.; Hanaki, T.; Nakasone, M.; Kitagawa, Y.; Koike, T.; Matsura, T. Estrogen receptor-mediated effect of delta-tocotrienol prevents neurotoxicity and motor deficit in the MPTP mouse model of Parkinson’s disease. Neurosci. Lett. 2016, 610, 117–122. [Google Scholar] [CrossRef]
- Lan, J.; Jiang, D.H. Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J. Neural Transm. 1997, 104, 469–481. [Google Scholar] [CrossRef]
- Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 1993, 328, 176–183. [Google Scholar] [CrossRef]
- Morens, D.M.; Grandinetti, A.; Waslien, C.I.; Park, C.B.; Ross, G.W.; White, L.R. Case-control study of idiopathic Parkinson’s disease and dietary vitamin E intake. Neurology 1996, 46, 1270–1274. [Google Scholar] [CrossRef]
- Hellenbrand, W.; Boeing, H.; Robra, B.P.; Seidler, A.; Vieregge, P.; Nischan, P.; Joerg, J.; Oertel, W.H.; Schneider, E.; Ulm, G. Diet and Parkinson’s disease. II: A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 1996, 47, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Scheider, W.L.; Hershey, L.A.; Vena, J.E.; Holmlund, T.; Marshall, J.R.; Freudenheim, J.L. Dietary antioxidants and other dietary factors in the etiology of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1997, 12, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Checkoway, H.; Franklin, G.M.; Beresford, S.; Smith-Weller, T.; Swanson, P.D. Dietary factors in Parkinson’s disease: The role of food groups and specific foods. Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 21–27. [Google Scholar] [CrossRef]
- Johnson, C.C.; Gorell, J.M.; Rybicki, B.A.; Sanders, K.; Peterson, E.L. Adult nutrient intake as a risk factor for Parkinson’s disease. Int. J. Epidemiol. 1999, 28, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.M.; Hernan, M.A.; Chen, H.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002, 59, 1161–1169. [Google Scholar] [CrossRef]
- de Rijk, M.C.; Breteler, M.M.; den Breeijen, J.H.; Launer, L.J.; Grobbee, D.E.; van der Meche, F.G.; Hofman, A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch. Neurol. 1997, 54, 762–765. [Google Scholar] [CrossRef]
- Etminan, M.; Gill, S.S.; Samii, A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: A meta-analysis. Lancet Neurol. 2005, 4, 362–365. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Swomley, A.M.; Forster, S.; Keeney, J.T.; Triplett, J.; Zhang, Z.; Sultana, R.; Butterfield, D.A. Abeta, oxidative stress in Alzheimer disease: Evidence based on proteomics studies. Biochim. Biophys. Acta 2014, 1842, 1248–1257. [Google Scholar] [CrossRef] [Green Version]
- Sultana, R.; Butterfield, D.A. Role of oxidative stress in the progression of Alzheimer’s disease. J. Alzheimer Dis. JAD 2010, 19, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huebbe, P.; Jofre-Monseny, L.; Boesch-Saadatmandi, C.; Minihane, A.M.; Rimbach, G. Effect of apoE genotype and vitamin E on biomarkers of oxidative stress in cultured neuronal cells and the brain of targeted replacement mice. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2007, 58, 683–698. [Google Scholar]
- Saito, Y.; Nishio, K.; Akazawa, Y.O.; Yamanaka, K.; Miyama, A.; Yoshida, Y.; Noguchi, N.; Niki, E. Cytoprotective effects of vitamin E homologues against glutamate-induced cell death in immature primary cortical neuron cultures: Tocopherols and tocotrienols exert similar effects by antioxidant function. Free Radic. Biol. Med. 2010, 49, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Regner, L.; Mett, J.; Stahlmann, C.P.; Schorr, P.; Nelke, C.; Streidenberger, O.; Stoetzel, H.; Winkler, J.; Zaidan, S.R.; et al. Tocotrienol Affects Oxidative Stress, Cholesterol Homeostasis and the Amyloidogenic Pathway in Neuroblastoma Cells: Consequences for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagl, S.; Grewal, R.; Ciobanu, I.; Helal, A.; Khayyal, M.T.; Muller, W.E.; Eckert, G.P. Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer’s disease. J. Alzheimer Dis. JAD 2015, 43, 927–938. [Google Scholar] [CrossRef]
- Selvaraju, T.R.; Khaza’ai, H.; Vidyadaran, S.; Abd Mutalib, M.S.; Vasudevan, R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn. J. Basic Med. Sci. 2014, 14, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Damanhuri, H.A.; Rahim, N.I.A.; Nasri, W.N.W.; Tan, J.K.; Makpol, S.; Mazlan, M.; Tooyama, I.; Ngah, W.Z.W. Tocotrienol-Rich Fraction Supplementation Modulates Antioxidant Enzymes Activity and Reduces DNA Damage in APPswe/PS1dE9 Alzheimer’s Disease Mouse Model. Sains Malays. 2016, 45, 1363–1370. [Google Scholar]
- Hagl, S.; Berressem, D.; Grewal, R.; Sus, N.; Frank, J.; Eckert, G.P. Rice bran extract improves mitochondrial dysfunction in brains of aged NMRI mice. Nutr. Neurosci. 2016, 19, 1–10. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Bishnoi, M.; Chopra, K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol. Biochem. Behav. 2009, 93, 183–189. [Google Scholar] [CrossRef]
- Schloesser, A.; Esatbeyoglu, T.; Piegholdt, S.; Dose, J.; Ikuta, N.; Okamoto, H.; Ishida, Y.; Terao, K.; Matsugo, S.; Rimbach, G. Dietary Tocotrienol/γ-Cyclodextrin Complex Increases Mitochondrial Membrane Potential and ATP Concentrations in the Brains of Aged Mice. Oxid. Med. Cell. Longev. 2015, 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Mangialasche, F.; Kivipelto, M.; Mecocci, P.; Rizzuto, D.; Palmer, K.; Winblad, B.; Fratiglioni, L. High plasma levels of vitamin E forms and reduced Alzheimer’s disease risk in advanced age. J. Alzheimer Dis. JAD 2010, 20, 1029–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangialasche, F.; Solomon, A.; Kareholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Westman, E.; Kivipelto, M.; Muehlboeck, J.S.; Cecchetti, R.; Baglioni, M.; Tarducci, R.; Gobbi, G.; Floridi, P.; Soininen, H.; et al. Classification and prediction of clinical diagnosis of Alzheimer’s disease based on MRI and plasma measures of alpha-/gamma-tocotrienols and gamma-tocopherol. J. Intern. Med. 2013, 273, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Hagl, S.; Kocher, A.; Schiborr, C.; Eckert, S.H.; Ciobanu, I.; Birringer, M.; El-Askary, H.; Helal, A.; Khayyal, M.T.; Frank, J.; et al. Rice bran extract protects from mitochondrial dysfunction in guinea pig brains. Pharm. Res. 2013, 76, 17–27. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Hopman, W.M.; Harrison, M.B.; Coo, H.; Friedberg, E.; Buchanan, M.; VanDenKerkhof, E.G. Associations between chronic disease, age and physical and mental health status. Chronic Dis. Can. 2009, 29, 108–116. [Google Scholar]
- Khoo, T.L.; Halim, A.S.; Zakaria, Z.; Mat Saad, A.Z.; Wu, L.Y.; Lau, H.Y. A prospective, randomised, double-blinded trial to study the efficacy of topical tocotrienol in the prevention of hypertrophic scars. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2011, 64, e137–e145. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bentinger, M.; Savu, O.; Moshfegh, A.; Sunkari, V.; Dallner, G.; Swiezewska, E.; Catrina, S.B.; Brismar, K.; Tekle, M. Mono-epoxy-tocotrienol-alpha enhances wound healing in diabetic mice and stimulates in vitro angiogenesis and cell migration. J. Diabetes Complicat. 2017, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Kania, M.; Danikiewicz, W.; Kaczorowska, E.; Wojcik, J.; Brismar, K.; Dallner, G.; Chojnacki, T.; Swiezewska, E.; Tekle, M. Effects of various squalene epoxides on coenzyme Q and cholesterol synthesis. Biochim. Biophys. Acta 2014, 1841, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Elsy, B.; Khan, A.A.; Maheshwari, V. Therapeutic potential of d-δ-tocotrienol rich fraction on excisional skin wounds in diabetic rats. Dermatol. Online 2017, 8, 376. [Google Scholar] [CrossRef]
- Elsy, B.; Khan, A.A.; Maheshwari, V. Effect of vitamin E isoforms on the primary intention skin wound healing of diabetic rats. Derm. Online 2017, 8, 369–375. [Google Scholar] [CrossRef]
- Musalmah, M.; Nizrana, M.Y.; Fairuz, A.H.; NoorAini, A.H.; Azian, A.L.; Gapor, M.T.; Wan Ngah, W.Z. Comparative effects of palm vitamin E and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids 2005, 40, 575–580. [Google Scholar] [CrossRef]
- Nurlaily, A.; Azian, A.; Musalmah, M. Tocotrienol-rich fraction formulation enhances wound healing in streptozotocin-induced diabetic rats. Med. Health 2011, 6, 234. [Google Scholar]
- Pierpaoli, E.; Orlando, F.; Cirioni, O.; Simonetti, O.; Giacometti, A.; Provinciali, M. Supplementation with tocotrienols from Bixa orellana improves the in vivo efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in a mouse model of infected wound. Phytomed. Int. J. Phytother. Phytopharm. 2017, 36, 50–53. [Google Scholar] [CrossRef]
- Arffah, K.S.; Yusur, S.M.; Rasool, A.H.G.; Halim, A.S.; Zilfalil, B.A. Effect of Tocotrienol Rich Fraction (TRF) on Fibroblasts from Normal and Hypertrophic Scar Tissues in Vitro. Int. Med. J. 2009, 16, 247–250. [Google Scholar]
- Mahirah, Y.S.; Halim, S.A.; Fazila, N.S.; Lau, H.Y.; Shaharum, S.; Rasool, A.H.; Rosline, H. Effect Of Tocotrienol On Keratinocytes And Fibroblasts In Normal And Hypertrophic Scar Cultures. Malays. J. Med. Sci. 2006, 13, 216. [Google Scholar]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Szabo, C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br. J. Pharmacol. 2009, 156, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Shoji, A.; Gu, N.; Joo, E.; Li, S.; Adachi, T.; Yamazaki, H.; Yasuda, K.; Kondoh, T.; Tsuda, K. γ-Tocotrienol attenuates TNF-α-induced changes in secretion and gene expression of MCP-1, IL-6 and adiponectin in 3T3-L1 adipocytes. Mol. Med. Rep. 2012, 5, 905–909. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol inhibits nuclear factor-κB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef]
- Candiracci, M.; Justo, M.L.; Castano, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q. γ-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPβ and NF-κB in macrophages. J. Nutr. Biochem. 2013, 24, 1146–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. 2014, 39, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yagiz, Y.; Xu, C.; Lu, J.; Chung, S.; Marshall, M.R. Muscadine grape seed oil as a novel source of tocotrienols to reduce adipogenesis and adipocyte inflammation. Food Funct. 2015, 6, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, W.; Okla, M.; Kang, I.; Moreau, R.; Chung, S. Suppression of NLRP3 inflammasome by γ-tocotrienol ameliorates type 2 diabetes. J. Lipid Res. 2016, 57, 66–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lim, Y. Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J. Nutr. Biochem. 2018, 57, 77–85. [Google Scholar] [CrossRef]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef]
- Haghighat, N.; Vafa, M.; Eghtesadi, S.; Heidari, I.; Hosseini, A.; Rostami, A. The Effects of Tocotrienols Added to Canola Oil on Microalbuminuria, Inflammation, and Nitrosative Stress in Patients with Type 2 Diabetes: A Randomized, Double-blind, Placebo-controlled Trial. Int. J. Prev. Med. 2014, 5, 617–623. [Google Scholar]
- Kuhad, A.; Bishnoi, M.; Tiwari, V.; Chopra, K. Suppression of NF-κB signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009, 92, 251–259. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFkB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Siddiqui, S.; Rashid Khan, M.; Siddiqui, W.A. Comparative hypoglycemic and nephroprotective effects of tocotrienol rich fraction (TRF) from palm oil and rice bran oil against hyperglycemia induced nephropathy in type 1 diabetic rats. Chem.-Biol. Interact. 2010, 188, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Matnor, N.A.; Iyer, A.; Brown, L. A Regenerative Antioxidant Protocol of Vitamin E and α-Lipoic Acid Ameliorates Cardiovascular and Metabolic Changes in Fructose-Fed Rats. Evid.-Based Complement. Altern. Med. 2011, 2011, 120801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, S.; Ahsan, H.; Khan, M.R.; Siddiqui, W.A. Protective effects of tocotrienols against lipid-induced nephropathy in experimental type-2 diabetic rats by modulation in TGF-β expression. Toxicol. Appl. Pharmacol. 2013, 273, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Abdul-Rahman, M.; Al-Wahaibi, N.; Mohammed, J. Tocotrienol-rich fraction from palm oil prevents oxidative damage in diabetic rats. Sultan Qaboos Univ. Med. J. 2014, 14, e95–e103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafa, M.; Haghighat, N.; Moslehi, N.; Eghtesadi, S.; Heydari, I. Effect of Tocotrienols enriched canola oil on glycemic control and oxidative status in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. J. Res. Med Sci. Off. J. Isfahan Univ. Med Sci. 2015, 20, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wan Nazaimoon, W.M.; Sakinah, O.; Gapor, A.; Khalid, B.A.K. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr. Res. 1996, 16, 1901–1911. [Google Scholar] [CrossRef]
- Burdeos, G.C.; Nakagawa, K.; Abe, T.; Kimura, F.; Miyazawa, T. Tocotrienol modulates crucial lipid metabolism-related genes in differentiated 3T3-L1 preadipocytes. Food Funct. 2014, 5, 2221–2227. [Google Scholar] [CrossRef]
- Zhao, L.; Ha, J.H.; Okla, M.; Chung, S. Activation of autophagy and AMPK by gamma-tocotrienol suppresses the adipogenesis in human adipose derived stem cells. Mol. Nutr. Food Res. 2014, 58, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.J.; Huang, G.Y.; Ng, L.T. γ-Tocotrienol induced cell cycle arrest and apoptosis via activating the Bax-mediated mitochondrial and AMPK signaling pathways in 3T3-L1 adipocytes. Food Chem. Toxicol. 2013, 59, 501–513. [Google Scholar] [CrossRef]
- Ali, S.F.; Nguyen, J.C.; Jenkins, T.A.; Woodman, O.L. Tocotrienol-Rich Tocomin Attenuates Oxidative Stress and Improves Endothelium-Dependent Relaxation in Aortae from Rats Fed a High-Fat Western Diet. Front. Cardiovasc. Med. 2016, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torabi, S.; Yeganehjoo, H.; Shen, C.L.; Mo, H. Peroxisome proliferator-activated receptor γ down-regulation mediates the inhibitory effect of d-δ-tocotrienol on the differentiation of murine 3T3-F442A preadipocytes. Nutr. Res. 2016, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wan Nazaimoon, W.M.; Khalid, B.A.K. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays. J. Pathol. 2002, 24, 77–82. [Google Scholar] [PubMed]
- Chen, C.W.; Cheng, H.H. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Nutr. 2006, 136, 1472–1476. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef]
- Chia, L.L.; Jantan, I.; Chua, K.H.; Lam, K.W.; Rullah, K.; Aluwi, M.F. Effects of Tocotrienols on Insulin Secretion-Associated Genes Expression of Rat Pancreatic Islets in a Dynamic Culture. Front. Pharm. 2016, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Hor, C.P.; Fung, W.Y.; Ang, H.A.; Lim, S.C.; Kam, L.Y.; Sim, S.W.; Lim, L.H.; Choon, W.Y.; Wong, J.W.; Ch’ng, A.S.H.; et al. Efficacy of Oral Mixed Tocotrienols in Diabetic Peripheral Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 444–452. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.K.; Kontto, J.P.; Mannisto, S.; Albanes, D.; Virtamo, J. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur. J. Clin. Nutr. 2011, 65, 590–597. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Dang, B.T.; Geny, C.; Blanchard, P.; Rouger, C.; Tonnerre, P.; Charreau, B.; Rakolomalala, G.; Randriamboavonjy, J.I.; Loirand, G.; Pacaud, P.; et al. Advanced glycation inhibition and protection against endothelial dysfunction induced by coumarins and procyanidins from Mammea neurophylla. Fitoterapia 2014, 96, 65–75. [Google Scholar] [CrossRef]
- Betik, A.C.; Aguila, J.; McConell, G.K.; McAinch, A.J.; Mathai, M.L. Tocotrienols and Whey Protein Isolates Substantially Increase Exercise Endurance Capacity in Diet -Induced Obese Male Sprague-Dawley Rats. PLoS ONE 2016, 11, e0152562. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.W.; Ma, C.Y.; Cheng, H.H.; Chen, Y.Y.; Lai, M.H. A rice bran oil diet improves lipid abnormalities and suppress hyperinsulinemic responses in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Clin. Biochem. Nutr. 2009, 45, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Abdul Nasir, N.A.; Agarwal, R.; Vasudevan, S.; Tripathy, M.; Alyautdin, R.; Mohd Ismail, N. Effects of topically applied tocotrienol on cataractogenesis and lens redox status in galactosemic rats. Mol. Vis. 2014, 20, 822–835. [Google Scholar]
- Abdul Nasir, N.A.; Agarwal, R.; Sheikh Abdul Kadir, S.H.; Vasudevan, S.; Tripathy, M.; Iezhitsa, I.; Mohammad Daher, A.; Ibrahim, M.I.; Mohd Ismail, N. Reduction of oxidative-nitrosative stress underlies anticataract effect of topically applied tocotrienol in streptozotocin-induced diabetic rats. PLoS ONE 2017, 12, e0174542. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comitato, R.; Nesaretnam, K.; Leoni, G.; Ambra, R.; Canali, R.; Bolli, A.; Marino, M.; Virgili, F. A novel mechanism of natural vitamin E tocotrienol activity: Involvement of ERbeta signal transduction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E427–E437. [Google Scholar] [CrossRef] [PubMed]
- Wali, V.B.; Bachawal, S.V.; Sylvester, P.W. Endoplasmic reticulum stress mediates gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis Int. J. Program. Cell Death 2009, 14, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Wali, V.B.; Bachawal, S.V.; Sylvester, P.W. Suppression in mevalonate synthesis mediates antitumor effects of combined statin and gamma-tocotrienol treatment. Lipids 2009, 44, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Hsieh, T.C.; Wu, J.M. Growth inhibition of human MDA-mB-231 breast cancer cells by delta-tocotrienol is associated with loss of cyclin D1/CDK4 expression and accompanying changes in the state of phosphorylation of the retinoblastoma tumor suppressor gene product. Anticancer Res. 2008, 28, 2641–2647. [Google Scholar]
- Ahmed, R.A.; Alawin, O.A.; Sylvester, P.W. gamma-Tocotrienol reversal of epithelial-to-mesenchymal transition in human breast cancer cells is associated with inhibition of canonical Wnt signalling. Cell Prolif. 2016, 49, 460–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loganathan, R.; Selvaduray, K.R.; Nesaretnam, K.; Radhakrishnan, A.K. Tocotrienols promote apoptosis in human breast cancer cells by inducing poly(ADP-ribose) polymerase cleavage and inhibiting nuclear factor kappa-B activity. Cell Prolif. 2013, 46, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.B.; Lim, S.W.; Loh, H.S. Synergistic Apoptotic Effects of Tocotrienol Isomers and Acalypha wilkesiana on A549 and U87MG Cancer Cells. Trop. Life Sci. Res. 2018, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Loh, H.S.; Ting, K.N.; Bradshaw, T.D.; Zeenathul, N.A. Antiproliferation and induction of caspase-8-dependent mitochondria-mediated apoptosis by beta-tocotrienol in human lung and brain cancer cell lines. Biomed. Pharmacother. 2014, 68, 1105–1115. [Google Scholar] [CrossRef]
- Lim, S.W.; Loh, H.S.; Ting, K.N.; Bradshaw, T.D.; Zeenathul, N.A. Cytotoxicity and apoptotic activities of alpha-, gamma- and delta-tocotrienol isomers on human cancer cells. BMC Complement. Altern. Med. 2014, 14, 469. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, K.; Virgona, N.; Harada, K.; Kido, W.; Yano, Y.; Ando, A.; Hagiwara, K.; Yano, T. A redox-silent analogue of tocotrienol acts as a potential cytotoxic agent against human mesothelioma cells. Life Sci. 2009, 84, 650–656. [Google Scholar] [CrossRef]
- Yano, Y.; Satoh, H.; Fukumoto, K.; Kumadaki, I.; Ichikawa, T.; Yamada, K.; Hagiwara, K.; Yano, T. Induction of cytotoxicity in human lung adenocarcinoma cells by 6-O-carboxypropyl-alpha-tocotrienol, a redox-silent derivative of alpha-tocotrienol. Int. J. Cancer 2005, 115, 839–846. [Google Scholar] [CrossRef]
- McAnally, J.A.; Gupta, J.; Sodhani, S.; Bravo, L.; Mo, H. Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp. Biol. Med. 2007, 232, 523–531. [Google Scholar]
- Abdul Rahman Sazli, F.; Jubri, Z.; Abdul Rahman, M.; Karsani, S.A.; Md Top, A.G.; Wan Ngah, W.Z. Gamma-tocotrienol treatment increased peroxiredoxin-4 expression in HepG2 liver cancer cell line. BMC Complement. Altern. Med. 2015, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Burdeos, G.C.; Ito, J.; Eitsuka, T.; Nakagawa, K.; Kimura, F.; Miyazawa, T. delta and gamma tocotrienols suppress human hepatocellular carcinoma cell proliferation via regulation of Ras-Raf-MEK-ERK pathway-associated upstream signaling. Food Funct. 2016, 7, 4170–4174. [Google Scholar] [CrossRef]
- Sakai, M.; Okabe, M.; Tachibana, H.; Yamada, K. Apoptosis induction by gamma-tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 2006, 17, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Okabe, M.; Yamasaki, M.; Tachibana, H.; Yamada, K. Induction of apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anticancer Res. 2004, 24, 1683–1688. [Google Scholar] [PubMed]
- Hiura, Y.; Tachibana, H.; Arakawa, R.; Aoyama, N.; Okabe, M.; Sakai, M.; Yamada, K. Specific accumulation of gamma- and delta-tocotrienols in tumor and their antitumor effect in vivo. J. Nutr. Biochem. 2009, 20, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Kurata, T.; Nakagawa, K.; Miyazawa, T. Synergistic inhibition of cancer cell proliferation with a combination of delta-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun. 2014, 453, 606–611. [Google Scholar] [CrossRef]
- Constantinou, C.; Hyatt, J.A.; Vraka, P.S.; Papas, A.; Papas, K.A.; Neophytou, C.; Hadjivassiliou, V.; Constantinou, A.I. Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr. Cancer 2009, 61, 864–874. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006, 346, 447–453. [Google Scholar] [CrossRef]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. delta-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, e12576. [Google Scholar] [CrossRef]
- Kaneko, S.; Sato, C.; Shiozawa, N.; Sato, A.; Sato, H.; Virgona, N.; Yano, T. Suppressive Effect of Delta-Tocotrienol on Hypoxia Adaptation of Prostate Cancer Stem-like Cells. Anticancer Res. 2018, 38, 1391–1399. [Google Scholar] [CrossRef]
- Xu, W.; Mi, Y.; He, P.; He, S.; Niu, L. gamma-Tocotrienol Inhibits Proliferation and Induces Apoptosis via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells. Molecules 2017, 22, 1299. [Google Scholar] [CrossRef] [Green Version]
- Comitato, R.; Guantario, B.; Leoni, G.; Nesaretnam, K.; Ronci, M.B.; Canali, R.; Virgili, F. Tocotrienols induce endoplasmic reticulum stress and apoptosis in cervical cancer cells. Genes Nutr. 2016, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.N.; Yap, W.N.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Evidence of gamma-tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutr. Cancer 2009, 61, 357–366. [Google Scholar] [CrossRef]
- Marzagalli, M.; Moretti, R.M.; Messi, E.; Marelli, M.M.; Fontana, F.; Anastasia, A.; Bani, M.R.; Beretta, G.; Limonta, P. Targeting melanoma stem cells with the Vitamin E derivative delta-tocotrienol. Sci. Rep. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Montagnani Marelli, M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E delta-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Lim, K.H.; Kam, T.S.; Loh, H.S. Synergistic cytotoxic effects of combined delta-tocotrienol and jerantinine B on human brain and colon cancers. J. Ethnopharmacol. 2016, 184, 107–118. [Google Scholar] [CrossRef]
- Sun, W.G.; Song, R.P.; Wang, Y.; Zhang, Y.H.; Wang, H.X.; Ge, S.; Liu, J.R.; Liu, L.X. gamma-Tocotrienol-Inhibited Cell Proliferation of Human Gastric Cancer by Regulation of Nuclear Factor-kappaB Activity. J. Agric. Food Chem. 2019, 67, 441–451. [Google Scholar] [CrossRef]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Nakagawa, K.; Miyazawa, T. A Combination of delta-Tocotrienol and Ferulic Acid Synergistically Inhibits Telomerase Activity in DLD-1 Human Colorectal Adenocarcinoma Cells. J. Nutr. Sci. Vitaminol. 2016, 62, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Husain, K.; Francois, R.A.; Hutchinson, S.Z. Vitamin E delta-tocotrienol levels in tmor and pancreatic tissue of mice after oral administration. Pharmacology 2009, 83, 157–163. [Google Scholar] [CrossRef]
- Hassen, S.; Ali, N.; Chowdhury, P. Molecular signaling mechanisms of apoptosis in hereditary non-polyposis colorectal cancer. World J. Gastrointest. Pathophysiol. 2012, 3, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. gamma-Tocotrienol-induced endoplasmic reticulum stress and autophagy act concurrently to promote breast cancer cell death. Biochem. Cell Biol. 2015, 93, 306–320. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Berberine and musculoskeletal disorders: The therapeutic potential and underlying molecular mechanisms. Phytomed. Int. J. Phytother. Phytopharm. 2019. [Google Scholar] [CrossRef]
- Tham, S.Y.; Loh, H.S.; Mai, C.W.; Fu, J.Y. Tocotrienols Modulate a Life or Death Decision in Cancers. Int. J. Mol. Sci. 2019, 20, 372. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fan, J.; Ju, D. 15-Neurotoxicity concern about the brain targeting delivery systems. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 377–408. [Google Scholar] [CrossRef]
- Ananthula, S.; Parajuli, P.; Behery, F.A.; Alayoubi, A.Y.; El Sayed, K.A.; Nazzal, S.; Sylvester, P.W. Oxazine derivatives of gamma- and delta-tocotrienol display enhanced anticancer activity in vivo. Anticancer Res. 2014, 34, 2715–2726. [Google Scholar]
- Huang, Y.; Wu, R.; Su, Z.Y.; Guo, Y.; Zheng, X.; Yang, C.S.; Kong, A.N. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. J. Nutr. Biochem. 2017, 40, 155–163. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M.P. delta-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef] [Green Version]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Algayadh, I.G.; Dronamraju, V.; Sylvester, P.W. Role of Rac1/WAVE2 Signaling in Mediating the Inhibitory Effects of gamma-Tocotrienol on Mammary Cancer Cell Migration and Invasion. Biol. Pharm. Bull. 2016, 39, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Kurisu, S.; Suetsugu, S.; Yamazaki, D.; Yamaguchi, H.; Takenawa, T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 2005, 24, 1309–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.Y.; Skretting, G.; Jing, Y.; Sun, H.; Sandset, P.M.; Sun, L. Hypoxia influences stem cell-like properties in multidrug resistant K562 leukemic cells. Blood Cells Mol. Dis. 2013, 51, 177–184. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, J.; Wei, W.; Shi, M.; Xin, B.; Zhang, T.; Shen, X. Hypoxia regulates ABCG2 activity through the activivation of ERK1/2/HIF-1alpha and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol. Ther. 2016, 17, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.K.; Then, S.M.; Mazlan, M.; Raja Abdul Rahman, R.N.; Jamal, R.; Wan Ngah, W.Z. Gamma-tocotrienol acts as a BH3 mimetic to induce apoptosis in neuroblastoma SH-SY5Y cells. J. Nutr. Biochem. 2016, 31, 28–37. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M.P. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, C.B.; Andersen, R.F.; Steffensen, K.D.; Adimi, P.; Jakobsen, A. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharm. Res. 2019, 141, 392–396. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Selvaduray, K.R.; Abdul Razak, G.; Veerasenan, S.D.; Gomez, P.A. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: A pilot clinical trial. Breast Cancer Res. BCR 2010, 12, R81. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Toll-like Receptor as a Molecular Link between Metabolic Syndrome and Inflammation: A Review. Curr. Drug Targets 2019, 20, 1264–1280. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Ryu, H.; Bahadduri, P.; Swaan, P.W.; Ratan, R.R.; Sen, C.K. Molecular basis of vitamin E action: Tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J. Biol. Chem. 2003, 278, 43508–43515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciffolilli, S.; Wallert, M.; Bartolini, D.; Krauth, V.; Werz, O.; Piroddi, M.; Sebastiani, B.; Torquato, P.; Lorkowski, S.; Birringer, M.; et al. Human serum determination and in vitro anti-inflammatory activity of the vitamin E metabolite alpha-(13’-hydroxy)-6-hydroxychroman. Free Radic. Biol. Med. 2015, 89, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Kumalaningsih, S.; Wijana, S.; Santoso, I. Antioxidative Effect of Tocotrienol Rich Fraction from Palm Fatty Acid Distillate on Oxidative Stress. Food Public Health 2013, 3, 130–136. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, P.W.; Shah, S.J. Mechanisms mediating the antiproliferative and apoptoic effects of Vitamin E in mammary cancer cells. Front. Biosci. 2005, 10, 699–709. [Google Scholar] [CrossRef]
- Birringer, M.; EyTina, J.H.; Salvatore, B.A.; Neuzil, J. Vitamin E analogues as inducers of apoptosis: Structure-function relation. Br. J. Cancer 2003, 88, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- Irias-Mata, A.; Sus, N.; Flory, S.; Stock, D.; Woerner, D.; Podszun, M.; Frank, J. alpha-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of alpha- and gamma-tocopherols and -tocotrienols in cultured liver cells. Redox Biol. 2018, 19, 28–36. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- McIntyre, B.S.; Briski, K.P.; Tirmenstein, M.A.; Fariss, M.W.; Gapor, A.; Sylvester, P.W. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 2000, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular basis of vitamin E action: Tocotrienol potently inhibits glutamate-induced Pp60c-Src kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendich, A.; Machlin, L.J. Safety of oral intake of vitamin E. Am. J. Clin. Nutr. 1988, 48, 612–619. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Nurshazwani, Y.; Shuid, A.N.; Muhammad, N.; Mohamed, N. Subacute and subchronic toxicity studies of palm vitamin E in mice. J. Pharmacol. Toxicol. 2011, 6, 166–173. [Google Scholar]
- Schurks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ 2010, 341, c5702. [Google Scholar] [CrossRef] [Green Version]











| Researcher (Year) | Cell Type | Treatment & Dose | Treatment Duration | Findings |
|---|---|---|---|---|
| Parker et al. (1993) [39] | Mammalian cells (HepG2 cells) supplemented with 7% lipoptotein-depleted serum in RPMI 1640 for 16 h to induce cholesterol genesis | T3 (10 µM) | 4 h | HMGCR: ↓ |
| Burdeos et al. (2013) [40] | Hepa 1-6 cells | γT3 (15 µM) | 24 h | TG: ↓, FAS: ↓, SREBP: ↓, SCD1: ↓, CPT1A: ↑, TC: ↔ |
| Burdeos et al. (2012) [41] | HepG2 cells | γT3 (10–15 µM) | 24 h | Cellular accumulation of TG (especially in the fat-loaded medium): ↓ |
| Song & DeBose-Boyd (2006) [44] | SV 589 cells (an immortalized line of human fibroblasts expressing SV-40 large T-antigen) | αT3 (10 µM) | 5 h | Did not have the function as γT3 and δT3 |
| γT3 (10 µM) | More selective than δT3 | |||
| δT3 (10 µM) | Ubiquitination and degradation of HMGCR: ↑, SREBP processing: ↓ | |||
| Theriault et al. (1999) [46] | HepG2 cells (HB 8065) supplemented with 7% lipoptotein-depleted serum in RPMI-1640 for 16 h to induce cholesterol genesis | γT3 | 6 h | Apo-B degradation: ↑, Apo-B translocation into the ER lumen: ↓, number of secreted Apo-B containing lipoprotein particles: ↓ |
| Zaiden et al. (2010) [47] | HepG2 cells supplemented with 7% lipoptotein-depleted serum in RPMI-1640 for 16 h to induce cholesterol genesis | γδT3 (20 µM) | 16 h | Diacylglycerol O-acyltransferase 2 (DGAT2), APOB100, SREBP1/2 and HMGCR: ↓, biosynthesis of TG, TC and VLDL: ↓, efflux of LDL through LDL receptor expression: ↑ |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Chicken model | |||||
| Qureshi et al. (1996) [48] | White Leghorn female chicken (n = 10, 2 weeks old) | 120 nmol/g αTF | Oral | 26 days | αTF attenuated the inhibition of HMGCR by γT3 Effective preparation of T3 mixture to exhibit anti-cholesterol effects contains 15–20% αTF and approximately 60% γT3 or δT3 Less effective preparation contains 30% αTF and approximately 45% γT3 or δT3 |
| γT3 (60 nmol/g) + αTF (60 nmol/g) | |||||
| γT3 (72 nmol/g) + αTF (48 nmol/g) | |||||
| γT3 (84 nmol/g) + αTF (36 nmol/g) | |||||
| γT3 (96 nmol/g) + αTF (24 nmol/g) | |||||
| γT3 (120 nmol/g) | |||||
| Qureshi & Peterson (2001) [49] | White Leghorn female chickens (n = 32, 2 weeks old) | TRF25 (d-P21-T3 + d-P25-T3) (50 ppm) | Oral | 4 weeks | TC: ↓, TG: ↓, LDL-C: ↓, HDL-C: ↓, HMGCR activity: ↓, ApoA-1: ↔, Apo-B: ↓, thromboxane B2: ↓, platelet factor 4: ↓ |
| TRF25 (d-P21-T3 + d-P25-T3) (50 ppm) + lovastatin (50 ppm) | TC: ↓, TG: ↓, LDL-C: ↓, HDL-C: ↔, HMGCR activity: ↓, ApoA-1: ↔, Apo-B: ↓, thromboxane B2: ↓, platelet factor 4: ↓ | ||||
| Yu et al. (2006) [53] | White Leghorn female chickens fed with cholesterol-free corn soy diet (5 weeks old) | TRF, γT3 or δT3 (50–2000 ppm) | Oral | 4 weeks | TC: ↓, LDL-C: ↓ |
| αTF (50–2000 ppm) | TC: ↔, LDL-C: ↔ | ||||
| αT3 (50–500 ppm) | TC: ↓, LDL-C: ↓ | ||||
| Qureshi et al. (2011) [50] | White Leghorn female chicken fed with corn-soy diet (n = 24; one day old) | δT3 (50 ppm/kg diet) | Oral | 4 weeks | TNF-α: ↓, NO: ↓, TC: ↓, LDL-C: ↓, lipid elevating impact of dexamethasone: ↓, TG lowering impact of riboflavin: ↑ |
| Hansen et al. (2015) [26] | 48 Hy-Line W-36 laying hens | 2000 mg/kg T3 | Oral | 7 weeks | TC: ↔, HDL-C: ↔, TG: ↔ |
| 2000 mg/kg T3 + 200 mg/kg αTF | |||||
| 2000 mg/kg T3 + 1000 mg/kg αTF | |||||
| Swine model | |||||
| Qureshi et al. (1991) [51] | Normolipemic swine | TRF (10–20% αTF, 15–20% αT3, 30–35% γT3, 20–25% δT3) (50 μg/g) | Oral | 42 days | TC: ↓, HDL-C: ↔, LDL-C: ↓, ApoA-1: ↔, Apo B: ↓, thromboxane B2: ↓, platelet factor 4: ↓, HMGCR activity in adipose tissue: ↓ |
| Genetically hypercholesterolemic | |||||
| Qureshi et al. (2001) [52] | Genetically hypercholesterolemic swine | TRF25 (50 µg) | Oral | 6 weeks | TC: ↓, HDL-C: ↔, LDL-C: ↓, ApoA-1: ↔, Apo B: ↓, TG: ↓, thromboxane B2: ↓, platelet factor 4: ↓, glucose: ↓, glucagon: ↓, insulin: ↑, HMGCR activity: ↓, cholesterol 7α-hydroxylase: ↔ |
| γT3 (50 µg) | |||||
| d-P21-T3 (50 µg) | |||||
| d-P25-T3 (50 µg) | |||||
| Rat model | |||||
| Watkins et al. (1993) [54] | Male Wistar rats on atherogenic diet | γT3 (50 mg/kg) | Oral | 6 weeks | TC: ↓, LDL-C: ↓, VLDL: ↓, TG: ↓, TBARS: ↓, fatty acid hydroperoxides: ↓ |
| αTF (500 mg/kg) | |||||
| Kaku et al. (1999) [55] | Male Sprague-Dawley rats (4 weeks old) | Mixed T3 (18.9% αT3, 5.5% βT3, 55.9% γT3, 16.7% δT3) (0.002% of diet) | Oral | 3 weeks | TG: ↓, TC: ↔, phospholipids: ↔ |
| Iqbal et al. (2003) [59] | Rats treated with chemical carcinogen DMBA to induce mammary carcinogenesis and hypercholesterolemia | TRF (10 mg/kg) | Oral | 6 months | TC: ↓, LDL-C: ↓, HMGCR activity & protein mass: ↓, severity & extent of neoplastic transformation in mammary glands: ↓, plasma & mammary ALP activity: ↓, GST activity: ↓ |
| Minhajuddin et al. (2005) [56] | Male albino rats fed with atherogenic diet | TRF (0–50 mg/kg) | Oral | 1 weeks | TG: ↓, TC: ↓, LDL: ↓, TBARS: ↓, conjugated dienes: ↓, HMGCR: ↓, optimum dose: 8 mg/kg |
| Budin et al. (2009) [58] | Male Sprague-Dawley rats induced by STZ | TRF (200 mg/kg) | Oral | 8 weeks | Glucose: ↓, HbA1c: ↓, TC: ↓, LDL-C: ↓. TG: ↓, HDL-C: ↑, SOD: ↑, MDA & 4-HNE (plasma and aorta): ↓, DNA damage: ↓ |
| Zaiden et al. (2010) [47] | LDLr-deficient mice (strain B6; 129s7-Ldlrtm1Her/J, LDLr−/−) | γδT3 (50 mg/kg) | Oral | 4 weeks | TC ↓, TG: ↓, HDL-C: ↔, LDL-C: ↓ |
| γδT3 + αTF (50 mg/kg) | |||||
| Burdeos et al. (2012) [41] | Male F344 rats fed with high fat diet | αTF (10 mg/kg) | Oral | 3 weeks | TG, TC, phospholipid & PLOOH (liver & plasma): ↔ |
| Rice bran T3 (1.9% αTF, 2.1% γTF, 2.0% δTF, 31.4% αT3, 50.5% γT3, 0.4% δT3) (5 mg/day) | TG, TC, phospholipid & PLOOH (liver & plasma): ↔ | ||||
| Rice bran T3 (1.9% αTF, 2.1% γTF, 2.0% δTF, 31.4% αT3, 50.5% γT3, 0.4% δT3) (10 mg/day) | TG, phospholipid & PLOOH (liver & plasma): ↓, TC (liver & plasma): ↔ | ||||
| Cheng et al. (2017) [57] | Male post-weaning Sprague-Dawley rats fed with high fat diet (8 weeks) to induce MetS | TRF (60 mg/kg) | Oral | 4 weeks | TC: ↓, non-HDL-C: ↓, TG: ↔ |
| Guinea pigs | |||||
| Khor et al. (1995) [60] | Male albino Harley guinea pigs | T3 isolated from palm fatty acid distillate (5 or 8 mg/day) | i.p. | 6 days | HMGCR: ↓ |
| T3 isolated from palm fatty acid distillate (10 mg/day) | HMGCR: ↔ | ||||
| Khor & Ng (2000) [63] | Male albino guinea pigs | T3 (10 mg/kg/day) | i.p. | 6 days | HMGCR: ↓ |
| T3 (10 mg/kg/day) + αTF (5 mg/kg/day) | HMGCR: ↓ (lesser) | ||||
| Khor et al. (2002) [64] | Male and female albino Harley guinea pigs | PVE (1 mg T3) (200 µL) | i.p. | 6 days | HMGCR: ↓, cholesterol 7α-hydroxylase: ↓ |
| PVE (3 mg T3) (200 µL) | |||||
| PVE (5 mg T3) (200 µL) | |||||
| PVE (1 mg αTF) (200 µL) | HMGCR: ↓, cholesterol 7α-hydroxylase: ↓ (less effective than T3) | ||||
| PVE (3 mg αTF) (200 µL) | |||||
| PVE (5 mg αTF) (200 µL) | HMGCR: ↓, cholesterol 7α-hydroxylase: ↓ | ||||
| PVE (20 mg αTF) (200 µL) | HMGCR: ↔, cholesterol 7α-hydroxylase: ↔ | ||||
| PVE (50 mg αTF) (200 µL) | HMGCR: ↑ | ||||
| Khor & Chieng (1997) [61] | Male Golden Syrian hamsters | Palm oil triacylglycerol fraction +72 ppm TF | Oral | 45 days | TC: ↑, LDL-C: ↑, HDL-C: ↑ |
| POTG + 162 ppm T3 | TC: ↓, LDL-C: ↔, HDL-C: ↔, TG: ↔ | ||||
| POTG + 1000 ppm T3 | TC: ↑, LDL-C: ↑, HDL-C: ↑ | ||||
| Raederstorff et al. (2002) [62] | Male Golden Syrian hamsters | Mixed T3 [29.5% αT3, 3.3% βT3, 41.4% γT3, 0.1% δT3, 25.1% others (mainly 9.9% γTF)] (39 mg/kg/day) | Oral | 4 weeks | Plasma TC: ↓, LDL-C: ↓ (starting from 2 weeks), TG: ↔, HDL-C: ↔ |
| Mixed T3 [29.5% αT3, 3.3% βT3, 41.4% γT3, 0.1% δT3, 25.1% others (mainly 9.9% γTF)] (263 mg/kg/day) | Plasma TC: ↓, LDL-C: ↓ (reduced significantly after 4 weeks), TG: ↔, HDL-C: ↔ | ||||
| γT3 [0.6% αT3, 7.0% βT3, 86.1% γT3, 0.1% δT3, 6.2% others (mainly 1.2% γTF)] (23 mg/kg/day) | Plasma TC: ↔, LDL-C: ↔, TG: ↔ | ||||
| γT3 [0.6% αT3, 7.0% βT3, 86.1% γT3, 0.1% δT3, 6.2% others (mainly 1.2% γTF)] (58 mg/kg/day) | Plasma TC: ↓, LDL-C: ↓ (reduced significantly after 4 weeks), TG: ↔, HDL-C: ↔ | ||||
| γT3 [0.6% αT3, 7.0% βT3, 86.1% γT3, 0.1% δT3, 6.2% others (mainly 1.2% γTF)] (263 mg/kg/day) | Plasma TC: ↓, LDL-C: ↓ (reduced significantly after 4 weeks), TG: ↔, HDL-C: ↔ | ||||
| Khor & Ng (2000) [63] | Male hamsters | 30 ppm αTF | Oral | 45 days | HMGCR: ↓ |
| 81 ppm αTF | HMGCR: ↑ | ||||
| Salman Khan et al. (2011) [45] | Male Golden hamsters | Tocomin (6.4% αT3, 1.0% βT3, 10.2% γT3, 3.2% δT3, 5.7% αTF) (10 mg/day) | Oral | 10 days | Plasma lipids: ↓, lipoprotein lipids: ↓, TC: ↓, Apo-B: ↓, small dense-LDL: ↓, LDL-C: ↓, inhibition of HMG-CoA reductase: δT3 > γT3 > βT3 > αT3 |
| Researcher (Year) | Study Population | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Positive effect | |||||
| Qureshi et al. (1991) [65] | Hypercholesterolemic subjects (n = 25; aged 30–60 years) | PVE (15–20% TF, 12–15% T3, 35–40% γT3, 25–30% δT3) (200 mg/day) | Oral | 4 weeks | TC: ↓, LDL-C: ↓, Apo-B: ↓, thromboxane B2: ↓, platelet factor 4: ↓, glucose: ↓ |
| Qureshi et al. (1995) [66] | Hypercholesterolemic subjects on AHA Step-1 diet for 4 or 8 weeks (n = 36; aged 40.6 ± 12.1 years) | PVE (40 mg αTF, 48 mg αT3, 112 mg γT3, 60 mg δT3) (220 mg) | Oral | 4 weeks | TC: ↓, ApoA-1: ↔, HDL-C: ↔ |
| γT3 (200 mg) | Oral | 4 weeks | TC: ↓, Apo-B: ↓, ex vivo thromboxane B2: ↓, ApoA-1: ↔, HDL-C: ↔ | ||
| Qureshi et al. (2001) [67] | Hypercholesterolemic subjects on AHA Step-1 diet since days 36–70) (n = 14; aged 40.14 ± 8.98 years) | Lovastatin (10 mg/day) | Oral | Day 71–105 | TC ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓ |
| Lovastatin (10 mg/day) + TRF (50 mg/day) | Day 106–140 | TC: ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓ | |||
| Lovastatin (10 mg/day) + αTF (50 mg/day) | Day 141–175 | TC: ↔, LDL-C: ↔, HDL-C/LDL-C ratio: ↑ | |||
| Hypercholesterolemic subjects on AHA Step-1 diet since days 36–70) (n = 14; aged: 42.57 ± 6.36 years) | TRF25 (50 mg/day) | Oral | Day 71–105 | TC ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓ | |
| Lovastatin (10 mg/day) + TRF25 (50 mg/day) | Day 106–140 | TC: ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓ | |||
| Lovastatin (10 mg/day) + αTF (50 mg/day) | Day 141–175 | TC: ↔, LDL-C: ↔, HDL-C/LDL-C ratio: ↑ | |||
| Qureshi et al. (2002) [68] | Hypercholesterolemic subject on AHA Step-1 diet starting day 36 (n = 90; men: aged <50 years, women: aged <40 years) | TRF25 (25 mg/day) | Oral | 35 days | TC: ↓, LDL-C: ↓ |
| TRF25 (50 mg/day) | TC: ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓, Apo A-1: ↑, HDL-C: ↑ | ||||
| TRF25 (100 mg/day) | TC: ↓, LDL-C: ↓, Apo-B: ↓, TG: ↓ (maximum decrease), Apo A-1: ↑, HDL-C: ↑ | ||||
| TRF25 (200 mg/day) | TC: ↔, LDL-C: ↔, Apo-B: ↔, TG: ↔ | ||||
| Zaiden et al. (2010) [47] | Hypercholesterolemic subjects (n = 20; aged 25–55 years) | γδT3 (120 mg/day) | Oral | 8 weeks | TG: ↓, VLDL: ↓, chylomicron: ↓. TC: ↔, LDL-C: ↔, HDL-C: ↔ |
| Yuen et al. (2011) [69] | Hypercholesterolemic subjects (n = 32; aged 31–53 years) | Mixed T3 (22.9 IU αTF, 30.8% αT3, 56.4% γT3, 12.8% δT3) (300 mg/day) | Oral | 6 months | TC ↓, LDL-C: ↓ |
| Ajuluchukwu et al. (2007) [70] | Patients with hypercholesterolemia and one additional cardiovascular risk factor (n = 34) | T3 (500 mg/day) | Oral | 4 weeks | Serum TC: ↓, LDL-C: ↓, HDL-C: ↔, TG: ↔ |
| αTF (500 mg/day) | Serum TC: ↔, LDL-C: ↔, HDL-C: ↔, TG: ↔ | ||||
| Heng et al. (2015) [71] | Adults with MetS (n = 70, aged 20–60 years) | Mixed T3 (61.1 mg αTF, 61.52 mg αT3, 112.80 mg γT3, 25.86 mg δT3) (400 mg/day) | Oral | 16 weeks | Serum TC: ↓, LDL-C: ↓, HDL-C: ↓, TG: ↔, FBG: ↔, IL-6: ↓, TNF-α: ↓, TC/HDL-C ratio: ↑ |
| Baliarsingh et al. (2005) [72] | Type 2 diabetic subjects (n = 19) | TRF (3 mg/kg) | Oral | 60 days | Total lipid: ↓, TC: ↓, LDL-C: ↓ |
| Daud et al. (2013) [73] | Subjects undergoing chronic hemodialysis (n = 81) | TRF (20 mg αTF, 30.18 mg αT3, 5.30 mg βT3, 41.66 mg γT3, 12.86 mg δT3) (90 mg/day) | Oral | 16 weeks | Normalized plasma TG: ↓, normalized plasma HDL-C: ↑, Apo-A 1: ↑, CETP: ↓ |
| Tan et al. (1991) [74] | Healthy volunteers (Preliminary: n = 9, follow up: n = 22) | PVE (18 mg TF, 32 mg T3, 240 mg palm olein) | Oral | 30 days | TC: ↓, LDL-C: ↓, TG: ↔, HDL-C: ↔ |
| Chin et al. (2011) [75] | Young healthy volunteers (n = 31; aged 35–49 years) | TRF (48 mg αTF, 70.4 mg αT3, 4.8 mg βT3, 57.6 mg γT3, 33.6 mg δT3) (160 mg/day) | Oral | 6 months | TC: ↔, TG: ↔, HDL-C: ↔, LDL-C: ↔, HDL-C/TC: ↔, SOD: ↔, GPx: ↔, CAT: ↔, protein carbonyl contents: ↔, AGEs: ↔, MDA: ↔ |
| Old healthy volunteers (n = 31; aged >50 years) | TC: ↔, TG: ↔, HDL-C: ↑, LDL-C: ↔, HDL-C/TC: ↑, SOD: ↓, CAT: ↓, GPx: ↑, CAT: ↔, protein carbonyl contents: ↓, AGEs: ↓, MDA: ↔ | ||||
| Heng et al. (2013) [76] | Young (n = 6; aged 34.6 ± 0.8 years) and old (n = 8; aged 49.5 ± 0.9 years) subjects | TRF (22% TF, 78% T3) (150 mg/day) | Oral | 6 months | Apo-A 1 precursor: ↑, Apo-E precursor: ↑, CRP precursor: ↓ |
| No effect | |||||
| Tomeo et al. (1995) [77] | Subjects with cerebrovascular disease (n = 50; aged 49–83 years) | TRF (16 mg αTF, 40 mg αT3, 40 mg γT3, 240 mg PVE) (300 mg) | Oral | 18 months | Apparent carotid atherosclerotic regression shown, TBARS: ↓, TC: ↔, LDL-C: ↔, TG: ↔, HDL-C: ↔ |
| Mensink et al. (1999) [78] | Men with mildly elevated lipid concentration (n = 40; aged 21–61 years) | Vitamin E concentrate rich in T3 (20 mg αTF, 35 mg T3) | Oral | 6 weeks | Serum LDL-C: ↔, HDL-C: ↔, TG: ↔, lipoprotein a: ↔, lipid peroxide: ↔, platelet aggregation velocity: ↔, maximum aggregation: ↔, thromboxane B2 formation: ↔ |
| O’Bryne et al. (2000) [79] | Healthy volunteers on AHA Step-1 diet for 12 weeks (n = 51) | α-tocotrienyl acetate (148 mg tocotrienyl acetate, 134 mg free T3) (250 mg/day) | Oral | 8 weeks (still on low fat diet) | In vitro LDL oxidative resistance: ↑, rate of LDL oxidation: ↓, serum LDL-C: ↔, Apo-B: ↔ |
| γ-tocotrienyl acetate (140 mg tocotrienyl acetate, 127 mg free T3) (250 mg/day) | |||||
| δ-tocotrienyl acetate (119 mg tocotrienyl acetate, 108 mg free T3) (250 mg/day) | |||||
| Mustad et al. (2002) [80] | Healthy subjects on AHA Step-1 diet for 7 weeks (n = 68, aged 25–65 years) | Mixed αT3 and γT3 (200 mg/day) | Oral | 28 days | Serum T3: ↑, lipid: ↔, glucose: ↔ |
| Pure γT3 (200 mg/day) | |||||
| P25 complex T3 (200 mg/day) | |||||
| Rasool et al. (2006) [81] | Healthy men (n = 36; aged 21–30 years) | T3-rich vitamin E (80 mg/day) | Oral | 2 months | Arterial compliance (pulse wave velocity & augmentation index): ↔, TC: ↔, LDL-C: ↔, aortic systolic BP: ↔, plasma total antioxidant status: ↔ |
| T3-rich vitamin E (160 mg/day) | Arterial compliance (pulse wave velocity & augmentation index): ↔, TC: ↔, LDL-C: ↔, aortic systolic BP: ↓, plasma total antioxidant status: ↔ | ||||
| T3-rich vitamin E (320 mg/day) | Arterial compliance (pulse wave velocity & augmentation index): ↔, TC: ↔, LDL-C: ↔, aortic systolic BP: ↓, plasma total antioxidant status: ↑ | ||||
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Myocardial ischemia perfusion rats model | |||||
| Das et al. (2008) [96] | Male Sprague-Dawley rats | αT3 (0.3 mg/kg) | Oral | 30 days | Cytochrome-C in mitochondrial fraction: ↑, p38 MAPKα binding with caveolin-1: ↔, Src and caveolin-1 interaction: ↔, caveolin-1 and HO-1 interaction: ↔, eNOS and caveolin-1 interaction: ↔, post ischemic 26S proteasome activity: ↔, Rpt5 and α3 subunit protein: ↔ |
| δT3 (0.3 mg/kg) | Cytochrome-C mitochondrial fraction: ↔, p38 MAPKα binding with caveolin-1: ↔, Src and caveolin-1 interaction: ↔, caveolin-1 and HO-1 interaction: ↔, eNOS and caveolin-1 interaction: ↑, post ischemic 26S proteasome activity: ↔, Rpt5 and α3 subunit protein: ↔ | ||||
| γT3 (0.3 mg/kg) | Cytochrome-C mitochondrial fraction: ↑, p38 MAPKα binding with caveolin-1: ↑, Src and caveolin-1 interaction: ↔, caveolin 1 and HO-1 interaction: ↓, eNOS and caveolin-1 interaction: ↔, post ischemic 26S proteasome activity: ↔, Rpt5 and α3 subunit protein: ↔ | ||||
| Das et al. (2012) [97] | Male and female adult New Zealand rabbits | αT3 (20 µmol/kg) | Oral | 4 weeks | TC: ↓, coronary flow: ↑, aortic flow: ↑, left ventricular developed pressure: ↑, infarct size: ↓, TGF-β, MMP-2, MMP-9: ↓, p-Akt: ↑, ER-α: ↑: ER-β: ↔, Spot 14: ↓, Forkhead box O4: ↑ |
| γT3 (20 µmol/kg) | |||||
| δT3 (20 µmol/kg) | TC: ↔, coronary flow: ↔, aortic flow: ↔, left ventricular developed pressure: ↔, infarct size: ↔, TGF-β, MMP-2, MMP-9: ↓ (female), p-Akt: ↑, ER-α: ↑: ER-β: ↔, Spot 14: ↔, Forkhead box O4 (female): ↑ | ||||
| Hyperhomocysteinemia rats model | |||||
| Kamisah et al. (2015) [99]; Norsidah et al. (2013) [93]; Norsidah et al. (2013) [94] | Male Wistar rats fed with high methionine diet (n = 42) | TRF (40.62% αTF acetate, 21.62% αTF, 10.71% αT3, 3.46% γTF, 18.08% γT3, and 5.49% δT3) (30 mg/kg diet) | Basal diet | 5 weeks | Plasma homocysteine level: ↔; S-adenosyl methionine (SAM)/S-adenosyl homocysteine (SAH) ratio: ↔; hepatic cystathionine: ↓; liver TBARS: ↔; aortic TBARS count: ↓; NO: ↔; aortic vascular cell adhesion protein 1 (VCAM-1) expression: ↔; intima-media thickness and intima media ratio: ↔; systolic BP: ↔; heart TBARS content: ↔; GPx: ↔; SOD: ↔; CAT: ↔ |
| TRF (40.62% αTF acetate, 21.62% αTF, 10.71% αT3, 3.46% γTF, 18.08% γT3, and 5.49% δT3) (60 mg/kg diet) | Plasma homocysteine level: ↔; SAM/SAH ratio: ↓; hepatic cystathionine: ↓; liver TBARS: ↓; aortic TBARS count: ↓; NO: ↓; aortic VCAM-1 expression: ↔; intima-media thickness and intima media ratio: ↔; systolic BP: ↔; heart TBARS content: ↓; GPx: ↑; SOD: ↔; CAT: ↔ | ||||
| TRF (40.62% αTF acetate, 21.62% αTF, 10.71% αT3, 3.46% γTF, 18.08% γT3, and 5.49% δT3) (150 mg/kg diet) | Plasma homocysteine level: ↓; SAM/SAH ratio: ↓; hepatic cystathionine: ↓; liver TBARS: ↓; aortic TBARS count: ↓; NO: ↓; aortic VCAM-1 expression: ↓; intima-media thickness and intima media ratio: ↔; systolic BP: ↔; heart TBARS content: ↓; GPx: ↑; SOD: ↔; CAT: ↔ | ||||
| MetS rat model | |||||
| Cheng et al. (2017) [57] | Male post-weaning Sprague-Dawley rats fed with high-fat diet (60% kcal) for 8 weeks (n = 21, aged 3 weeks old) | TRF—Tocovid (23.5% αT3, 43.2% γT3, 9.8% δT3, and 23.5% αTF) (60 mg/kg) | Oral | 4 weeks | Visceral adiposity: ↔, systolic and diastolic BP: ↓, oral glucose tolerance test (OGTT): ↔, antioxidant capacity: ↑, MPO hyperactivity: ↓, expression of receptor for advanced glycation end product (RAGE): ↓, HBA1c: ↓, AGEs: ↓, TG: ↑, TC: ↓, non-HDL-C: ↓, HDL-C: ↔, non-esterified fatty acid (NEFA): ↔, hepatic TG deposition: ↓, liver total lipid: ↔, PPARs: ↔, PPAR-α and PPAR-γ expression: ↔ |
| Wong et al. (2012) [102] | Male Wistar rats fed with high carbohydrate, high fat (n = 32, aged 9–10 weeks old) | TRF (31.9% αT3, 2.1% βT3, 24.8% γT3, 18.3% δT3 and 22.9% αTF) (120 mg/kg) | Oral | 8 weeks | Ventricular function: ↑, attenuated cardiac stiffness: ↑, glucose and insulin tolerance: ↑, left ventricular collagen deposition: ↓, liver enzymes (ALT and AST): ↓, inflammatory cell infiltration: ↓, fat vacuoles: ↓, balloon hepatocytes: ↓, plasma free fatty acid: ↓, TG: ↓ |
| Wong et al. (2017) [103] | Male Wistar rats fed with HCHF diet (n = 80, aged 9–10 weeks old) | 91.6% αTF (85 mg/kg) | Oral | 8 weeks | Collagen deposition: ↓, inflammatory cell infiltration: ↓, TC: ↔, NEFA: ↔, TG: ↔, liver enzymes (ALT and AST): ↓, lipid droplet: ↓, fasting plasma glucose: ↔, total fat mass: ↔, abdominal circumference: ↔, adiposity index: ↔, retroperitoneal and epididymal fat pads: ↔ |
| 90.7% αT3 (85 mg/kg) | |||||
| 95% γT3 (85 mg/kg) | Collagen deposition: ↓, inflammatory cell infiltration: ↓, normalised systolic BP, TC: ↔, NEFA: ↓, TG: ↔, liver enzymes (ALT and AST): ↓, lipid droplet: ↓, fasting plasma glucose: ↔, total fat mass: ↔, abdominal circumference: ↔, adiposity index: retroperitoneal and epididymal fat pads: ↔ | ||||
| 90% δT3 (85 mg/kg) | Collagen deposition: ↓, inflammatory cell infiltration: ↓, normalised systolic BP, TC: ↓, NEFA: ↓, TG: ↓ abdominal adiposity: ↓, liver enzymes (ALT and AST): ↓, lipid droplet: ↓, fasting plasma glucose: ↓, total fat mass: ↓, abdominal circumference: ↓, adiposity index: ↓, retroperitoneal and epididymal fat pads: ↓ | ||||
| Hypertension rats model | |||||
| Muharis et al. (2010) [104] | Male SHRs and male Wistar Kyoto rats injected with STZ (75 mg/kg, i.p.) for 8 weeks (aged 18 weeks old) | αTF (0.01 mg/mL) | Aortic rings incubated with extracts | 20 minutes | Radical scavenging capability: ↑, acetylcholine relaxation (aortic ring): ↑ |
| TRF (0.01 mg/mL) | |||||
| Newaz et al. (2003) [105] | Male SHRs (n = 33, aged 8–10 weeks old) and male Wistar Kyoto (WKY) rats (n = 17, aged 8–10 weeks old) | 90% γT3 (15 mg/kg) | Oral | 3 months | Systolic BP: ↓, nitrite level: ↑, MDA: ↓, nitric oxide synthase (NOS) activity negatively correlated with BP |
| 90% γT3 (30 mg/kg) | Systolic BP: ↓, nitrite level: ↑, MDA: ↓, NOS activity: ↑, NOS activity negatively correlated with BP | ||||
| 90% γT3 (150 mg/kg) | |||||
| Researcher (Year) | Study Design | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Mahdy et al. (2013) [106] | Randomized placebo-controlled double-blind clinical trial (n = 151; mean age: 25.94) | TRF (100 mg/day) | Oral | 12–16 weeks pregnancy until delivery | Relative risk of PIH = 0.36 (95% CI = 0.12–1.09): ↔, Relative risk of preeclampsia = 0.20 (95% CI = 0.02–1.66): ↔, clinical severity: ↔, hematological or biochemical indices: ↔, blood loss at delivery: ↓ |
| Rasool et al. (2006) [81] | Randomized blinded end-point, placebo-controlled clinical trial (36 males, aged 21–30 years old) | T3 rich vitamin E (26.2% αTF, 34.6% αT3, 24.63 γT3, 15% δT3) (80 mg/day) | Oral | 2 months | Plasma vitamin E level: ↑, augmentation index: ↔, plasma total antioxidant status: ↔, TC: ↔, LDL-C: ↔ |
| T3 rich vitamin E (26.2% αTF, 34.6% αT3, 24.63 γT3, 15% δT3) (160 mg/day) | Plasma vitamin E level: ↑, augmentation index: ↔, plasma total antioxidant status: ↔, TC: ↔, LDL-C: ↔ | ||||
| T3 rich vitamin E (26.2% αTF, 34.6% αT3, 24.63 γT3, 15% δT3) (320 mg/day) | Plasma vitamin E level: ↑, plasma total antioxidant status: ↑, TC: ↔, LDL-C: ↔ | ||||
| Mustad et al. (2002) [80] | Randomized, double-blind parallel design (68 healthy subjects: 39 males and 29 females, aged 25–65 years old) | αT3 plus γT3 extract from palm oil (Tocovid) (200 mg/day) | Oral | 7 weeks | Serum concentrations of T3: ↔, fasting total: ↔, LDL-C: ↔, glucose: ↔, no significant correlation between serum T3 concentrations and changes in fasting serum lipids or glucose and urinary 8-epi-PGF2α. |
| High γT3 extract from rice bran oil (Nutriene) (200 mg/day) | Serum concentrations of T3: ↑, fasting total: ↔, LDL-C: ↔, glucose: ↔, no significant correlation between serum T3 concentrations and changes in fasting serum lipids or glucose and urinary 8-epi-PGF2α. | ||||
| P25-complex extract from rice bran oil (EvolvE) (200 mg/day) | Serum concentrations of T3: ↔, fasting total: ↔, LDL-C: ↔, glucose: ↔, no significant correlation between serum T3 concentrations and changes in fasting serum lipids or glucose and urinary 8-epi-PGF2α. | ||||
| Rasool et al. (2008) [107] | Randomized placebo controlled, blinded end point clinical study (36 healthy male, aged <40 years old) | Tocovid (23.5% αTF, 23.54% αT3, 23.54% γT3, 43.16% δT3) (50 mg/day) | Oral | 2 months | Pulse wave velocity: ↔, augmentation index: ↓, BP: ↔, TC: ↔ LDL-C: ↔. |
| Tocovid (23.5% αTF, 23.54% αT3, 23.54% γT3, 43.16% δT3) (100 or 200 mg/day) | Pulse wave velocity: ↓, augmentation index: ↓, BP: ↔, TC: ↔ LDL-C: ↔. |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Rink et al. (2011) [109] | Mongrel canines (acute ischemic stroke induced by MCAO) (n = 20, aged 2.4 ± 0.9 years) | TRF (200 mg twice per day) | Oral (gel capsule) | 10 weeks | Cytotoxic edema at 1 h: ↓; protect fibre tract projection (hemisphere); relative connectivity of fibre tracts between the internal capsule and corona radiate: ↑; cerebrovascular collateral circulation: ↑; artery territory collateral score: ↑; gene expression of chloride intracellular channel 1 and tissue inhibitor of metalloproteinase-1 (TIMP-1): ↑; MMP-2 (cerebral cortex): ↓. |
| Khanna et al. (2013) [110] | C57BL/6 male mice, Harlan (transient 90 minutes focal cerebral ischemic induced by MCAO (n = 12, aged 5 weeks old) | αT3 (50 mg/kg) | Oral | 10 weeks | Rescued stroke-induced loss of miR-29b and minimized lesion size. |
| Park et al. (2011) [111] | C57BL/6 male Harlan Indianapolis rats (transient focal cerebral ischemia induced by MCAO) (n = 41, aged 5 weeks old) | αT3 (50 mg/kg) | Oral | 13 weeks | Brain αT3 level: ↑; attenuated hemispherical infarct volume; abundance of MRP1 positive cells at infarct hemisphere: ↑; miR199a-5p in infarct hemisphere: ↓; attenuated stroke-induced neurodegeneration; 4-HNE-positive cells: ↓; |
| Mishima et al. (2003) [112] | Male ddY mice with focal cerebral infarction induced by MCAO (n = 80) | αTF (0.2 & 2 mM) | i.v. | 24 h | Infarct volume: ↓, body temperature: ↔, BP; ↔ |
| αT3 (0.2 & 2 mM) | |||||
| γTF (0.2 & 2 mM) | |||||
| γT3 (0.2 & 2 mM) | Infarct volume: ↔, body temperature: ↔, BP; ↔ | ||||
| δTF (0.2 & 2 mM) | |||||
| δT3 (0.2 & 2 mM) | |||||
| Jiao et al. (2018) [116]; Shang et al. (2018) [117] | Male ICR mice with transient MCAO for 60 minutes (n = 119, aged 6 weeks old) | Tocovid (11.3% αTF, 12.4% αT3, 2.5% βT3, 19.2% γT3, 6.3% δT3) (200 mg/kg/day) | Oral | 1 month | Mice neurobehaviors: ↑, infarct volume: ↓, inflammatory markers (TNF-α, MCP-1, Iba-1): ↓, neurovascular units (MMP-9, immunoglobulin G and collagen IV): ↑, rotarod time: ↑, infarct volume: ↓, 4-HNE, nitrotyrosine and 8-OhdG: ↓, RAGE, chaperone-mediated autophagy and CML expressions: ↓, Nrf2 and MRP1: ↑, GSSG/GSH ratio: ↓, caspase-3 and LC3-II expressions: ↓ |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Soelaiman et al. (2012) [135] | Ovariectomized female Sprague-Dawley rats (n = 32, aged 4 months old) | Palm T3 (24.67% αT3, 38.955% γT3, 4.55% δT3, 20.11% αTF) (60 mg/kg) | Oral | 8 weeks | dLS/BS: ↑, sLS/BS: ↓, MS: ↑, MAR: ↑, BFR/BS: ↑, OCN: ↔, CTX-1: ↓ |
| Aktifanus et al. (2012) [141] | Ovariectomized female Sprague-Dawley rats (n = 32, aged 4 months old) | TRF (24.67% αT3, 38.955% γT3, 4.55% δT3, 20.11% αTF) (60 mg/kg) | Oral | 2 months | OCN: ↔, CTX-1: ↔, sLS: ↓, dLS: ↑, MS: ↔, MAR: ↑, BFR: ↑ |
| Chin et al. (2017) [137] | Ovariectomized female Sprague-Dawley rats (n = 40, aged 3 months old) | Annatto T3 (10% γT3, 90% δT3) (60 mg/kg) | Oral | 8 weeks | sLS: ↓, dLS: ↑, MS: ↑, MAR: ↑, BFR: ↑, BMP-2: ↑ |
| Muhammad et al. (2012) [136] | Ovariectomized female Wistar rats (n = 40, aged 3 months old) | Palm T3 (37.2% αT3, 39.1% γT3, 22.6% δT3) (60 mg/kg) | Oral | 4 weeks | BV/TV: ↑, Tb.Sp: ↓, Tb.N: ↑, Tb.Th: ↔, Oc.S: ↓, Ob.S: ↔ |
| αTF (60 mg/kg) | |||||
| Deng et al. (2014) [138] | Ovariectomized female C57BL/6 mice (n =56, aged 8 weeks old) | γT3 (100 mg/kg) | s.c. | 3 months | BMD: ↑, BV/TV: ↑, Tb.Th: ↑, Tb.N: ↑, Tb.Sp: ↓, Oc.N: ↓, Ob.N: ↑, MAR: ↑, BFR: ↑, OCN: ↑, CTX-1: ↓, RANKL: ↓, OPG: ↑, Osterix: ↑, Runx-2: ↑ |
| Abdul-Majeed et al. (2012) [139] | Ovariectomized female Sprague-Dawley rats (n =48, aged 3 months old) | δT3 (60 mg/kg) | Oral | 8 weeks | OCN: ↑, CTX-1: ↓, Ob.S: ↑, Oc.S: ↓, ES: ↓, OS: ↑, OV: ↑ |
| δT3 (60 mg/kg) + lovastatin (11 mg/kg) | |||||
| Abdul-Majeed et al. (2015) [140] | Ovariectomized female Sprague-Dawley rats (n = 48, aged 3 months old) | δT3 (60 mg/kg) | Oral | 8 weeks | BV/TV: ↑, Tb.N: ↑, Tb.Th: ↑, Tb.Sp: ↓, load: ↑, stress: ↑, strain: ↑, Young’s Modulus: ↑ |
| δT3 (60 mg/kg) + lovastatin (11 mg/kg) | |||||
| Mohamad et al. (2012) [142] | Ovariectomized female Sprague-Dawley rats (n = 32, aged 3 months old) | αTF (60 mg/kg) | Oral | 2 months | Stress: ↔, load: ↔, strain: ↔, Young’s Modulus: ↔ |
| T3-enriched fraction (73.9 mg/g αTF, 167.1 mg/g αT3, 41.1 mg/g βT3, 165.2 mg/g γT3, 98.5 mg/g δT3) (60 mg/kg) | Stress: ↑, load: ↔, strain: ↔, Young’s Modulus: ↔ | ||||
| Norazlina et al. (2000) [134] | Ovariectomized female Sprague-Dawley rats (n = 40, aged 3 months old) | αTF (30 mg/kg) | Oral | 8 months | BMD: ↔, calcium (femur & lumbar): ↔, ALP: ↑, tartrate-resistant acid phosphatase (TRAP): ↓ |
| PVE (24.82% αTF, 20.73% αT3, 26.68% γT3, 13.32% δT3) (30 mg/kg) | BMD: ↔, calcium (femur & lumbar): ↓, ALP: ↑, TRAP: ↔ | ||||
| PVE (24.82% αTF, 20.73% αT3, 26.68% γT3, 13.32% δT3) (60 mg/kg) | BMD: ↔, calcium (femur & lumbar): ↔, ALP: ↔, TRAP: ↔ | ||||
| Muhammad et al. (2013) [161] | Ovariectomized female Wistar rats (n = 32, aged 3 months old) | Palm T3 (37.2% αT3, 39.1% γT3, 22.6% δT3) (60 mg/kg) | Oral | 4 weeks | IL-1: ↓, IL-6: ↓, OCN: ↓, pyridinoline (serum): ↔ |
| Nazrun et al. (2008) [159] | Ovariectomized female Wistar rats (n = 64, aged 3 months old) | αTF (60 mg/kg) | Oral | 8 weeks | MDA: ↔, GPx: ↔, SOD: ↑, load: ↔, stress: ↔, stiffness: ↔, Young’s Modulus: ↔ |
| Palm T3 (30.7% αT3, 55.2% γT3, 14.1% δT3) (60 mg/kg) | MDA: ↓, GPx: ↑, SOD: ↑, load: ↔, stress: ↔, stiffness: ↔, Young’s Modulus: ↔ | ||||
| Ibrahim et al. (2014) [143] | Ovariectomized female Sprague-Dawley rats with fracture at metaphysis region of right tibia (n =48, aged: 3 months old) | Annatto T3 particles (60 mg/kg) | Single injection | - | BV/TVcallus: ↔, BMDcallus: ↔, load: ↑, stress: ↑, strain: ↔, Young’s Modulus: ↔ |
| Annatto T3 particles (60 mg/kg) + lovastatin particles (750 µg/kg) | BV/TVcallus: ↑, BMDcallus: ↑, load: ↑, stress: ↑, strain: ↔, Young’s Modulus: ↔ | ||||
| Ibrahim et al. (2015) [166] | Ovariectomized female Sprague-Dawley rats with fracture at metaphysis region of right tibia (n = 48, aged 3 months old) | Annatto T3 particles (60 mg/kg) | Single injection | - | OCN: ↔, BMP-2: ↔, VEGF-α: ↔, Runx-2: ↔, bone sialoprotein (BSP): ↔, TGF-β2: ↔, TGF-β3: ↔, collagen type II alpha 1 (COL2α1): ↔, FGF-23: ↔ |
| Annatto T3 particles (60 mg/kg) + lovastatin particles (750 µg/kg) | OCN: ↑, BMP-2: ↑, VEGF-α: ↑, Runx-2: ↑, BSP: ↔, TGF-β2: ↔, TGF-β3: ↔, COL2α1: ↔, FGF-23: ↔ | ||||
| Norazlina et al. (2002) [133] | Female Sprague-Dawley rats fed with VED diet (n = 40, aged 4 months old) | αTF (30 mg/kg) | Oral | 8 months | calcium (5th lumbar & femur): ↔ |
| PVE (24.82% αTF, 20.73% αT3, 26.68% γT3, 13.32% δT3) (30 mg/kg) | calcium (5th lumbar): ↑, calcium (femur): ↔ | ||||
| PVE (24.82% αTF, 20.73% αT3, 26.68% γT3, 13.32% δT3) (60 mg/kg) | calcium (5th lumbar): ↑, calcium (femur): ↑ | ||||
| Norazlina et al. (2004) [132] | Female Sprague-Dawley rats fed with VED diet (n = 40, aged 3 months old) | αT3 (60 mg/kg) | Oral | 3 months | PTH (serum): ↑, calcium (serum): ↔, calcium (femoral): ↔, DPD (urine): ↔, OCN: ↔ |
| αTF (60 mg/kg) |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Maniam et al. (2008) [160] | Normal male Sprague-Dawley rats (n = 56, aged 3 months old) | αTF (30 mg/kg) | Oral | 4 months | TBARS: ↔, GPx: ↔, SOD: ↔ |
| αTF (60 mg/kg) | |||||
| αTF (100 mg/kg) | |||||
| Palm T3 (30 mg/kg) | |||||
| Palm T3 (60 mg/kg) | |||||
| Palm T3 (100 mg/kg) | TBARS: ↓, GPx: ↑, SOD: ↔ | ||||
| Mehat et al. (2010) [144] | Normal male Sprague-Dawley rats (n = 32, aged 3 months old) | αTF (60 mg/kg) | Oral | 4 months | Oc.N: ↓, N.Ob: ↑, ES: ↓, OS: ↑, sLS/BS: ↓, dLS/BS: ↑, BFR: ↑, OV: ↔, MS: ↔, MAR: ↔ |
| δT3 (60 mg/kg) | Oc.N: ↓, N.Ob: ↑, ES: ↓, OS: ↑, sLS/BS: ↓, dLS/BS: ↑, BFR: ↑, OV: ↑, MS: ↑, MAR: ↑ | ||||
| γT3 (60 mg/kg) | |||||
| Shuid et al. (2010) [157] | Normal male Sprague-Dawley rats (n = 24, aged 3 months old) | αTF (60 mg/kg) | Oral | 4 months | BV/TV: ↑, Tb,Th: ↑, Tb.N: ↑, Tb.Sp: ↓, displacement: ↑, stress:↑, strain: ↑, load: ↔, stiffness: ↔ |
| γT3 (60 mg/kg) | BV/TV: ↑, Tb.Th: ↑, Tb.N: ↑, Tb.Sp: ↓, displacement: ↑, stress: ↑, strain: ↑, load: ↑, stiffness: ↑ | ||||
| Tennant et al. (2017) [151] | Male Sprague-Dawley rats (n = 19, aged 11 weeks old) | Adequate dietary αTF | Oral | 18 weeks | Bone mineral content, bone area & BMD: ↔, cross-sectional volume: ↔. cortical volume: ↔, Ct.Th: ↔, BV/TV: ↔, Tb.Th: ↔, Tb.N: ↔, Tb.Sp: ↔, mineralizing perimeter: ↔ MAR: ↔, BFR: ↔,OCN (serum): ↔, Runx-2: ↔, Sp7: ↔, OCN (gene): ↓, osteopontin: ↔, ALP: ↔, RANKL: ↔, CSF-1: ↔ |
| High dietary αTF (500 mg/kg) | |||||
| High dietary mixed Tocomin (250 mg/kg diet) | |||||
| Norazlina et al. (2007) [162] | Nicotine-induced osteoporotic male Sprague-Dawley rats (n = 24, aged 3 months old) | Palm T3 (60 mg/kg) | Oral | 3 months | IL-1: ↔, IL-6: ↓, OCN: ↔, calcium (femur): ↔ |
| αTF (60 mg/kg) | IL-1: ↑, IL-6: ↑, OCN: ↔, calcium (femur): ↔ | ||||
| Hermizi et al. (2009) [145] | Nicotine-induced osteoporotic male Sprague-Dawley rats (n = 49, age: 3-month-old) | T3-enhanced fraction (43% αT3, 31% γT3, 14% δT3, 12% other oils) (60 mg/kg) | Oral | 2 months | BV/TV: ↑, Tb.Th: ↑, Tb.N: ↔, MAR: ↑, BFR: ↑, sLS: ↓, Ob.S: ↑, Oc.S: ↓, ES: ↓ |
| γT3 (60 mg/kg) | BV/TV: ↑, Tb.Th: ↑, Tb.N: ↑, MAR: ↑, BFR: ↑, sLS: ↓, Ob.S: ↑, Oc.S: ↓, ES: ↓ | ||||
| Norazlina et al. (2010b) [156] | Nicotine-induced osteoporotic male Sprague-Dawley rats (n = 32, aged 3 months old) | T3 mixture (60 mg/kg) | Oral | 12 weeks | calcium (femoral): ↑, OPG and RANKL (serum): ↔, calcium (4th lumbar): ↔ |
| αTF (60 mg/kg) | |||||
| Norazlina et al. (2010a) [164] | Nicotine-induced osteoporotic male Sprague-Dawley (n = 35, aged 3 months old) | αTF (60 mg/kg) | Oral | 4 months | IL-1: ↓, PYD: ↔, OCN: ↔ |
| T3-enriched fraction (60 mg/kg) | |||||
| γT3 (60 mg/kg) | IL-1: ↓, PYD: ↓, OCN: ↑ | ||||
| Abukhadir et al. (2012) [165] | Nicotine-induced osteoporotic male Sprague-Dawley rats (n = 32, aged 3 months old) | PVE (60 mg/kg) | Oral | 4 months | BMP-2: ↑, OSX: ↑, Runx-2: ↑ |
| Ima-Nirwana & Fakhrurazi (2002) [154] | Adrenalectomized & dexamethasone-induced osteoporotic male Wistar rats (n = 70, age: 3-month-old) | PVE (20.73% αT3, 26.68% γT3, 13.32% δT3, 24.82% αTF) (60 mg/kg) | Oral | 8 weeks | BMD (whole body & regional): ↑, femoral length: ↑, calcium content: ↑ |
| Ima-Nirwana & Suhaniza (2004) [155] | Adrenalectomized & dexamethasone-induced osteoporotic male Sprague-Dawley rats (n = 42, age: 4-month-old) | γT3 (60 mg/kg) | Oral | 8 weeks | BMD: ↔, calcium content (femur): ↔, calcium content (4th lumbar): ↑ |
| Ahmad et al. (2005) [146] | Ferric nitrilotriacetate (2 mg/kg, i.p.)-induced osteoporotic male Wistar rats (n = 32, age: 4-week-old) | αTF (10 mg/kg) | Oral | 8 weeks | IL-1: ↔, IL-6: ↔, OCN: ↔ |
| αTF (30 mg/kg) | IL-1: ↔, IL-6: ↓, OCN: ↔, DPD: ↔ | ||||
| αTF (60 mg/kg) | IL-1: ↔, IL-6: ↓, OCN: ↔, DPD: ↔ | ||||
| αTF (100 mg/kg) | Ob.N: ↔, Oc.N: ↔, ES: ↔, BFR: ↔, BV/TV: ↔, Tb.Th: ↔, Tb.N: ↔, OCN: ↓, DPD: ↓, IL-1: ↔, IL-6: ↓ | ||||
| Palm T3 (30.7% αT3, 55.2% γT3 and 14.1% δT3) (10 mg/kg) | IL-1: ↔, IL-6: ↔, OCN: ↔, DPD: ↔ | ||||
| Palm T3 (30 mg/kg) | IL-1: ↔, IL-6: ↓, OCN: ↔, DPD: ↔ | ||||
| Palm T3 (60 mg/kg) | IL-1: ↓, IL-6: ↓, OCN: ↔, DPD: ↓ | ||||
| Palm T3 (100 mg/kg) | Ob.N: ↑, Oc.N: ↔, ES: ↓, BFR: ↑, BV/TV: ↑, Tb.Th: ↑, Tb.N: ↔, OCN: ↓, DPD: ↓, IL-1: ↓, IL-6: ↓ | ||||
| Ima-Nirwana et al. (2000) [153] | Orchidectomized male Wistar rats (n = 30, age: 3-month-old) | PVE (21.6% αT3, 27.7% γT3, 11% δT3, 15.3% palm olein, 24.4% αTF) (30 mg/kg) | Oral | 8 months | BMD: ↑, bone calcium content: ↔, ALP: ↔, TRAP: ↔ |
| Chin et al. (2014) [152] | Orchidectomized male Sprague-Dawley rats (n = 40, age: 3-month-old) | Annatto T3 (10% γT3, 90% δT3) (60 mg/kg) | Oral | 8 weeks | P1NP: ↔, TRACP 5b: ↔, Ob.S: ↑, Oc.S: ↓, ES: ↓, OS: ↑, OV: ↑ |
| Chin & Ima-Nirwana (2014) [147] | Orchidectomized male Sprague-Dawley rats (n = 40, age: 3-month-old) | Annatto T3 (10% γT3, 90% δT3) (60 mg/kg) | Oral | 8 weeks | OCN: ↔, CTX-1: ↔, BV/TV: ↑, Tb.N: ↑, Tb.Th: ↔, Tb.Sp: ↓, sLS: ↓, dLS: ↑, MS: ↔, MAR: ↔, BFR: ↔, ALPL: ↑, bone gamma-carboxyglutamate protein (BGLAP): ↔, COL1α1: ↑, β-catenin: ↑, RUNX-2: ↔, SP7: ↔, SPARC: ↔, integrin binding sialoprotein (IBSP): ↔, OPG: ↔ |
| Chin et al. (2016) [158] | Orchidectomized male Sprague-Dawley rats (n = 30, age: 3-month-old) | Annatto T3 (10% γT3, 90% δT3) (60 mg/kg) | Oral | 8 weeks | Calcium (serum): ↓, phosphate (serum): ↔, calcium (bone): ↑, load: ↔, stress: ↔, strain: ↔, extension: ↔ |
| Wong et al. (2018) [148]; Wong et al. (2018) [150]; Wong et al. (2019) [163] | MetS-induced osteoporotic male Wistar rats (n = 30, aged 12 weeks old) | Palm T3 (21.9% αTF, 24.7% αT3, 4.5% βT3, 36.9% γT3, 12.0% δT3) (60 mg/kg) | Oral | 20 weeks | Abdominal circumference: ↔, systolic & diastolic pressure: ↓, FBG: ↓, OGTT: ↔, TG: ↓, TC: ↓, HDL-C: ↔, LDL-C: ↓, fat mass: ↔, lean mass: ↔, percentage of fat: ↔, BV/TV: ↑, Tb.N: ↑, Tb.Sp: ↓, SMI: ↓, Tb.Th: ↔, Conn.D: ↔, bone calcium (femur): ↔, load: ↑, displacement: ↔, stiffness: ↑, stress: ↔, strain: ↔, Young’s Modulus: ↑, Ob.S: ↑, Oc.S: ↔, ES: ↔, OS: ↑, OV: ↔, sLS: ↓, dLS: ↔, MS: ↔, MAR: ↔, BFR: ↔, OCN: ↔, CTX-1: ↔, leptin: ↓, adiponectin: ↔, insulin: ↔, IL-6: ↓, IL-1α: ↔, IL-1β: ↔, TNF-α: ↔, OPG: ↔, RANKL: ↓, SOST: ↔, DKK-1: ↔, FGF-23: ↓, PTH: ↔ |
| Palm T3 (21.9% αTF, 24.7% αT3, 4.5% βT3, 36.9% γT3, 12.0% δT3) (100 mg/kg) | Abdominal circumference: ↔, systolic & diastolic pressure: ↓, FBG: ↓, OGTT: ↓, TG: ↓, TC: ↓, HDL-C: ↑, LDL-C: ↓, fat mass: ↔, lean mass: ↔, percentage of fat: ↔, BV/TV: ↑, Tb.N: ↑, Tb.Sp: ↓, SMI: ↓, Tb.Th: ↔, Conn.D: ↔, bone calcium (femur): ↔ load:↑, displacement: ↔, stiffness: ↔, stress: ↔, strain: ↔, Young Modulus: ↑, Ob.S: ↑, Oc.S: ↔, ES: ↑, OS: ↑, OV: ↔, sLS: ↔, dLS: ↔, MS: ↔, MAR: ↔, BFR: ↔, OCN: ↔, CTX-1: ↔, leptin: ↔, adiponectin: ↔, insulin: ↔, IL-1α: ↓, IL-1β: ↔, IL-6: ↓, TNF-α: ↔, OPG: ↔, RANKL: ↓, SOST: ↓, DKK-1: ↓, FGF-23: ↓, PTH: ↔ | ||||
| Wong et al. (2018) [149]; Wong et al. (2019) [163] | MetS-induced osteoporotic male Wistar rats (n = 30, aged 12 weeks old) | Annatto T3 (16% γT3, 84% δT3) (60 mg/kg) | Oral | 20 weeks | Abdominal circumference: ↔, systolic & diastolic pressure: ↓, FBG: ↓, OGTT: ↔, TG: ↓, TC: ↓, HDL-C: ↔, LDL-C: ↔, fat mass: ↔, lean mass: ↔, percentage of fat: ↔, BV/TV: ↑, Tb.N: ↑, Tb.Sp: ↓, SMI: ↓, Tb.Th: ↔, Conn.D: ↓, bone calcium (femur): ↔, load: ↔, displacement: ↔, stiffness: ↔, stress: ↔, strain: ↔, Young’s modulus: ↔, Ob.S: ↑, Oc.S: ↔, ES: ↔, OS: ↔, OV: ↔, sLS: ↓, dLS: ↔, MS: ↔, MAR: ↑, BFR: ↔, OCN: ↔, CTX-1: ↔, leptin: ↔, adiponectin: ↑, insulin: ↔, IL-1α: ↓, IL-1β: ↔, IL-6: ↓, TNF-α: ↔, OPG: ↔, RANKL: ↓, SOST: ↔, DKK-1: ↔, FGF-23: ↓, PTH: ↔ |
| Annatto T3 (16% γT3, 84% δT3) (100 mg/kg) | Abdominal circumference: ↔, systolic & diastolic pressure: ↓, FBG: ↓, OGTT: ↔, TG: ↓, TC: ↓, HDL-C: ↔, LDL-C: ↓, fat mass: ↔, lean mass: ↔, percentage of fat: ↔, BV/TV: ↑, Tb.N: ↑, Tb.Sp: ↓, SMI: ↓, Tb.Th: ↔, Conn.D: ↓, bone calcium content: ↔, load: ↑, displacement: ↔, stiffness: ↔, stress: ↔, strain: ↓, Young’s modulus: ↑, Ob.S: ↑, Oc.S: ↔, ES: ↔, OS: ↔, OV: ↔, sLS: ↔, dLS: ↔, MS: ↔, MAR: ↔, BFR: ↔, OCN: ↔, CTX-1: ↔, leptin: ↓, adiponectin: ↑, insulin: ↔, IL-1α: ↓, IL-1β: ↔, IL-6: ↓, TNF-α: ↔, OPG: ↔, RANKL: ↓, SOST: ↓, DKK-1: ↓, FGF-23: ↓, PTH: ↔ |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Radhakrishnan et al. (2014) [169] | CIA in female dark Agouti rats (n = 24, aged 10 weeks old) | γT3 (5 mg/kg) | Oral | 24 days | Hind paw thickness and edema: ↓, CRP: ↓, TNF-α: ↓, SOD: ↑, GSH: ↑ |
| Haleagrahara et al. (2014) [170] | CIA in female dark Agouti rats (n = 24, aged 6–10 weeks old) | δT3 (10 mg/kg) | Oral | 25 days | Paw edema (left & right hind limb joint): ↓, collagen-stimulated lymphocytes (proliferation): ↓, CRP: ↓ |
| Zainal et al. (2019) [172] | CIA in female dark Agouti rats (n = 30, aged 4–5 weeks old) | TRF (25% αTF, 75% T3) (30 mg/kg) | Oral | 18 days | Ankle circumference: ↓, swelling and redness of the joints: ↓, cartilage was smooth, number of cartilage cells: ↑, CRP: ↓, TNF-α: ↓, IL-1β: ↓, IL-6: ↓, BMD: ↑ |
| Chin et al. (2019) [171] | Sprague-Dawley male rats (n = 40, aged 3 months old) induced with monosodium iodoacetate | Annatto T3 (50, 100, 150 mg/kg) | Oral | 5 weeks | Joint histology scoring: ↓, cartilage oligomeric matrix protein in serum: ↓ |
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
|---|---|---|---|---|---|
| Stress-induced gastric ulcer model | |||||
| Ibrahim et al. (2011) [252] | WIRS-induced male Sprague-Dawley rats (n = 60) | PVE (60 mg/kg) | Oral | 28 days | Plasma acetylcholine: ↓, gastric contraction: ↓, amplitude of contraction: ↓, lesion index: ↓ |
| αTF (60 mg/kg) | |||||
| Fahami et al. (2011) [224] | WIRS-induced male Sprague-Dawley rats (n = 24) | TF (60 mg/kg) | Oral | 28 days | MDA: ↓, GPx: ↔, SOD: ↔, GSH/GSSG: ↑ |
| T3 (60 mg/kg) | MDA: ↓, GPx: ↓, SOD: ↔, GSH/GSSG: ↑ | ||||
| Kamisah et al. (2011) [223] | WIRS-induced male Sprague-Dawley rats (n = 60) | TRF (21% αTF, 4% γTF, 17% αT3, 33% γT3, 24% δT3) (60 mg/kg/day) | Oral | 28 days | Gastric lesions index: ↓, XO activity: ↓, TBARS: ↓ |
| αTF (60 mg/kg) | |||||
| Rodzian et al. (2013) [229] | WIRS-induced male Sprague-Dawley rats (n = 14) | T3 (90% δT3, 10% γT3) (60 mg/kg) | Oral | 28 days | Gastric lesions score: ↓, MDA: ↑, PGE2: ↔ |
| Nur Azlina et al. (2013) [221] | WIRS-induced male Wistar rats (n = 28) | T3 (44.8% d-γT3, 29.4% d-αT3, 10.8% d-δT3, and 5% d-βT3 (60 mg/kg) | Oral | 28 days | Gastric lesion index: ↓, gastric acid concentration: ↓, PGE2: ↑, COX-1: ↑, COX-2: ↓ |
| Nur Azlina et al. (2015) [230] | WIRS-induced male Wistar rats (n = 28) | T3 (60 mg/kg) | Oral | 28 days | Gastric lesion index: ↓, MDA: ↓, SOD activity: ↑, iNOS expression: ↓, NO: ↓, TNF-α: ↓, IL-1β: ↓ |
| Nur Azlina et al. (2017) [238] | WIRS-induced male Wistar rats (n = 28) | T3 (60 mg/kg) | Oral | 28 days | Gastric lesion index: ↓, VEGF: ↑, EGF: ↔, TGF-α: ↑, bFGF: ↑ |
| NSAIDs-induced gastric ulcer model | |||||
| Nafeeza et al. (2002) [240] | Aspirin-induced male Sprague-Dawley rats (n = 36) | TF (300 mg/kg diet) | Oral | 8 weeks | Gastric lesion index: ↓, gastric MDA content: ↓, adherent mucous concentration: ↔, gastric acid concentration: ↔ |
| TRF (80% T3 and 20% TF) (300 mg/kg diet) | |||||
| Saad et al. (2002) [244] | Indomethacin-induced male Sprague-Dawley rats (n = 48) | TRF (150 mg/kg diet) | Oral | 8 weeks | MDA: ↔, GSH: ↑, PGE2: ↔, gastric acid: ↔, gastric mucous concentration: ↔ |
| Jaarin et al. (2002) [242] | Aspirin-induced male Sprague-Dawley rats (n = 96) | PVE (60, 100, or 150 mg/kg diet) | Oral/orogastric | 4 weeks | Gastric lesion index: ↓, gastric MDA: ↓, gastric acid concentration: ↔ |
| TF (20, 30, or 50 mg/kg diet) | |||||
| Nafeeza & Kang (2005) [239] | Indomethacin-induced male Sprague-Dawley rats (n = 48) | Combination of TF-T3, TF-ubiquinone or T3-ubiquinone) | Oral | 28 days | Gastric lesion: ↔, lipid peroxidation: ↓, PGE2: ↑, gastric acid concentration: ↔, GSH/GSSG ratio: ↔ |
| Ohta et al. (2006) [245] | Compound C48/80-induced male Wistar rats (aged 6 weeks) | αTF (50 mg/kg) | Oral | 0.5 h after treatment with compound C48/80 | Gastric mucosal lesions: ↔, serotonin: ↔, histamine: ↔, gastric mucosal blood flow: ↔, vitamin E content: ↑, TBARS: ↓, MPO activity: ↔, XO activity: ↔, GPx activity: ↔, ascorbic acid & hexosamine: ↔ |
| αTF (100 mg/kg) | Gastric mucosal lesions: ↓, serotonin: ↔, histamine: ↔, gastric mucosal blood flow: ↔, vitamin E content: ↑, TBARS: ↓, MPO activity: ↓, XO activity: ↓, GPx activity: ↑, ascorbic acid & hexosamine: ↑ | ||||
| αTF (250 mg/kg) | |||||
| Fesharaki et al. (2006) [210] | Aspirin-induced male Wistar rats (n = 90) | Vitamin E (75 units) | Intragastric | 3, 6, 9, & 24 h | Gross & histological lesion: ↓, PGE2: ↑, MPO activity: ↓, XO activity: ↔, SOD activities: ↑, GSH: ↑, GPx: ↑ |
| Odabasoglu et al. (2008) [243] | Indomethacin-induced male Sprague-Dawley rats (n = 102) | αTF (100 mg/kg) | Oral | 6 h | Ulcer area: ↓, CAT: ↓, SOD: ↑, GST: ↓, glutathione reductase: ↑, GSH: ↑, MPO: ↓ |
| Jiang et al. (2009) [215] | Carrageenan-induced air-pouch inflammation male Wistar rats | Aspirin + γTF (33 mg/kg) | Oral | 3 days | PGE2: ↓, exudate volume: ↓, number of infiltrating immune cells: ↔, LDH: ↓, plasma & exudate γTF: ↑, 8-isoprostane: ↓ |
| Aspirin + αTF (33 mg/kg) | PGE2: ↔, exudate volume: ↔, number of infiltrating immune cells: ↔, LDH: ↔, plasma & exudate αTF: ↑, 8-isoprostane: ↔ | ||||
| Ethanol-induced gastric ulcer model | |||||
| Jaarin et al. (1999) [249] | Ethanol-induced male Sprague-Dawley rats (n = 56) | PVE (150 mg/kg diet) | Oral (PVE), i.p. (ranitidine) | 3 weeks | Mean ulcer index: ↓, gastric acid concentration: ↔, MDA: ↓, gastric mucous concentration: ↔ |
| PVE (150 mg/kg diet) + ranitidine (30 mg/kg) | Mean ulcer index: ↓, gastric acid concentration: ↓, MDA: ↓, gastric mucous concentration: ↔ | ||||
| Ismail et al. (1999) [250] | Ethanol-induced male Sprague-Dawley rats | PVE (150 mg/kg diet) | Oral | 3 weeks | Gastric acid concentration: ↔, MDA: ↓, PGE2: ↔ |
| Jaarin et al. (2000) [246] | Ethanol-induced male Sprague-Dawley rats (n = 42) | PVE (150 mg/kg diet) | Oral | 3 weeks | Mean ulcer index: ↓, MDA: ↓, gastric acid concentration: ↔, PGE2: ↔ |
| In Vitro Study | |||||
| Researcher (Year) | Cell Type | Treatment & Dose | Findings | ||
| Nakaso et al. (2014) [259] | Human dopaminergic neuroblastoma (SH-SY5Y) cells | αT3 (1 µM) | Cell viability: ↑, p-Akt: ↔, p-ERK: ↔ | ||
| βT3 (1 µM) | Cell viability: ↑, p-Akt: ↑, p-ERK: ↔ | ||||
| γT3 (1 µM) | Cell viability: ↑, p-Akt: ↑, p-ERK: ↑ | ||||
| δT3 (1 µM) | Cell viability: ↑, p-Akt: ↑, p-ERK: ↑ | ||||
| In Vivo Studies | |||||
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
| Lan et al. (1997) [261] | 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP)-induced neurodegeneration in male and female weanling C57BL/6 mice (21 ± 3 days old) | Iron (25 g/kg diet) + vitamin E (7 g/kg diet) | Oral | 30 days | Striatal iron concentration: ↓, GSSG: ↓, GSH: ↔, GSSG/GSH: ↓, MDA: ↓ |
| Nakaso et al. (2016) [260] | MPTP-induced neurodegeneration in C57BL/6 mice | αTF (100 μg/kg) | Oral | 4 days (day 2–6) | Wheel running activity: ↔, loss of dopaminergic neurons: ↓, time on rotarod: ↔ |
| δTF (100 μg/kg) | |||||
| αT3 (100 μg/kg) | Wheel running activity: ↔, loss of dopaminergic neurons: ↓, time on rotarod: ↑ | ||||
| δT3 (100 μg/kg) | Wheel running activity: ↑, loss of dopaminergic neurons: ↓, time on rotarod: ↔ | ||||
| Human Studies | |||||
| Researcher (Year) | Study Design | Study Population | Data Collection | Findings | |
| Parkinson study group (1993) [262] | Multicenter, controlled clinical trial | Untreated patients with Parkinson’s disease (n = 202) | Supplementation of TF (2000 IU/day) | TF did not delay the onset of disability associated with early otherwise unwanted Parkinson’s disease (HR = 0.91, 95% CI = 0.74–1.12) | |
| Moren et al. (1996) [263] | Nested case control study | Patients with idiopathic Parkinson’s disease (n = 84; aged 46–65 years) | Intake of dietary vitamin E | No beneficial effect of prior vitamin E intake on Parkinson’s disease occurrence (OR = 0.64, 95% CI = 0.35–1.17) | |
| Hellenbrand et al. (1996) [264] | Case control study | Patients with Parkinson’s disease (n = 342; mean age: 56.2 ± 6.7 years) | Past dietary habits | No association between Parkinson’s disease and vitamin E intake (OR = 0.92, 95% CI = 0.46–1.83) | |
| Scheider et al. (1997) [265] | Case control study | Male subjects with two cardinal signs of Parkinson’s disease (n = 126; aged 45–79 years) | Usual dietary intake (vitamin E) 20 years ago | No reduction in Parkinson’s disease risk was associated with higher vitamin E intakes (OR = 1.18, 95% CI = 0.47–2.98). | |
| Anderson et al. (1999) [266] | Case control study | Newly diagnosed idiopathic Parkinson’s disease in men and women (n = 103; aged 40–89 years) | Dietary habits during adult life | Intake of food containing vitamin E was not significantly related with Parkinson’s disease risk (OR = 1.21, 95% CI = 0.69–2.11) | |
| Johnson et al. (1999) [267] | Case control study | Patients with Parkinson’s disease (n = 126; aged ≥50 years) | Usual dietary intake | Weak positive association of Parkinson’s disease with vitamin E (OR = 1.25, 95% CI = 0.71–2.22) | |
| Zhang et al. (2002) [268] | Cohort study | Patients with Parkinson’s disease (n = 371; aged 30–55 years) | Dietary information on how often and amount of food | The risk of Parkinson’s disease was significantly reduced with high intake of dietary vitamin E (OR = 0.69, 95% CI = 0.49–0.98) | |
| de Rijk et al. (1997) [269] | Cross sectional study | Individual without dementia and participants with Parkinson’s disease (n = 5342; aged ≥55 years) | Intake of dietary antioxidants | High intake of dietary vitamin E protected against the occurrence of Parkinson’s disease (OR = 0.50, 95% CI = 0.20–0.90) | |
| In Vitro Studies | |||||
| Researcher (Year) | Cell Type | Treatment & Dose | Findings | ||
| Huebbe et al. (2007) [275] | Human neuroblastoma (SH-SY5Y) cells | αTF (25 µM) | Cell viability: ↑ (after treated with tBHP and BSO) | ||
| γTF (25 µM) | |||||
| αT3 (25 µM) | |||||
| γT3 (25 µM) | Cell viability: ↑ (after treated with tBHP) | ||||
| Saito et al. (2010) [276] | Primary cortical neurons isolated from cerebral cortex of Sprague-Dawley rat fetuses | αTF (0.25–2.5 µM) | Glutamate-induced cytotoxicity: ↓, cellular GSH: ↔, ROS: ↓, lipid hydroperoxide formation: ↓ | ||
| αT3 (0.25–2.5 µM) | |||||
| Grimm et al. (2016) [277] | Human neuroblastoma (SH-SY5Y) cells & neuro 2a (N2a) cells | αTF (10 µM) | TC: ↓, free cholesterol: ↓, ROS: ↔, hydrogen peroxide-induced ROS: ↓ | ||
| αT3 (10 µM) | TC: ↓, free cholesterol: ↓, ROS: ↓, hydrogen peroxide-induced ROS: ↓, Aβ production: ↑, Aβ degradation: ↓ | ||||
| Hagl et al. (2014) [278] | PC12 (from pheochromocytoma of rat adrenal medulla) and HEK293 (from human embryonic kidney) cells | Rice bran extract (0.02–0.5 mg/mL) | ATP level: ↑, mitochondrial membrane potential: ↑, mitochondrial respiration: ↑, citrate synthase activity: ↑, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α): ↑, Mfn1: ↓, Drp1: ↑, fis1: ↑, Opa1: ↔ | ||
| Selvaraju et al. (2014) [279] | Human glioblastoma (DBTRG-05MG) cells | TRF (100, 200 and 300 ng/mL) | Astrocytes’ viability: ↑, mitochondrial membrane potential: ↑, MDA level: ↓ | ||
| αTF (100, 200 and 300 ng/mL) | |||||
| In Vivo Studies | |||||
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
| Damanhuri et al. (2016) [280] | Double transgenic male mice B6C3-Tg (APPswe, PS1dE9)85Dbo/Mmjax with C57BL/6J genetic background | TRF (24% αTF, 27% αT3, 4% βT3, 32% γT3, 14% δT3) (200 mg/kg) | Oral | 6 months | SOD: ↓, GPx: ↔, CAT: ↑, DNA damage: ↓ |
| αTF (200 mg/kg) | SOD: ↓, GPx: ↔, CAT: ↔, DNA damage: ↓ | ||||
| Hagl et al. (2013) [289] | Male Dunkin Hartley guinea pigs | Rice bran extract (50 or 150 mg/kg) | Oral | 30 days | Respiration in brain mitochondria: ↑, resistance of brain cells against mitochondrial dysfunction: ↑, Drp1: ↑, fis1: ↑, Mfn1: ↔, Opa1: ↔, citrate synthase activity: ↑, cardiolipin: ↑, phosphatidylglycerol: ↑ |
| Hagl et al. (2016) [281] | Aged (18-month-old) female NMRI (Naval Medical Research Institute) mice | Rice bran extract (86 μg/g αTF, 71 μg/g βTF, 288 μg/g γTF, 93 μg/g δTF, 55 μg/g αT3, 2226 μg/g γT3, 266 μg/g δT3) (340 mg/kg) | Oral | 3 weeks | ATP concentration: ↑, basal mitochondrial membrane potential: ↑, complex I respiration: ↑, citrate synthase activity: ↑, PGC1α: ↑, complex V: ↑, Drp1: ↔, fis1: ↓, Mfn1: ↑, Opa1: ↔ |
| Tiwari et al. (2009) [282] | STZ-induced cognitive impairment and oxidative-nitrosative stress in male Wistar rats | αTF (100 mg/kg) | Oral | 20 days | Learning performance: ↑, time spent in target quadrant: ↑, retention transfer latency: ↓, spontaneous locomotor activity: ↔, AChE activity: ↓, MDA: ↓, GSH: ↑, SOD: ↑, CAT: ↑, nitrite: ↓ |
| T3 (mixture of αT3, βT3, γT3) (50 and 100 mg/kg) | |||||
| Schloesser et al. (2015) [283] | Male C57BL/6J mice | T3/γ-cyclodextrin complex diet (100 mg/kg diet) + αTF (20 mg/kg diet) | Oral | 24 weeks | ATP concentration: ↑, mitochondrial membrane potential: ↑, transcription factor A mitochondrial (TFAM) protein: ↑, PGC1α: ↔, Sod2: ↔, Hmox1: ↔, Gclm: ↔, Gpx4: ↔, Cat: ↔, proteasomal activity: ↔, BACE1: ↔ |
| Human Studies | |||||
| Researcher (Year) | Study Design | Study Population | Intervention | Duration | Findings |
| Mangialasche et al. (2010) [284] | Population-based cohort study | Dementia-free subjects (n = 232; aged 80+ years) | Plasma levels of vitamin E (αTF, βTF, γTF, δTF, αT3, βT3, γT3, δT3) | 6 years follow-up | High plasma levels of vitamin E were associated with a reduced risk of Alzheimer’s disease in advanced age |
| Devore et al. (2010) [288] | Population-based cohort study | Dementia-free subjects (n = 5395; aged 55+ years) | Dietary information at study baseline | 9.6 years follow-up | High intake of foods rich in vitamin E reduced long-term risk of dementia and Alzheimer’s disease. |
| Mangialasche et al. (2013) [286] | Longitudinal study | Patients with Alzheimer’s disease (n = 81; aged 75.1 ± 5.7 years) and MCI (n = 86; aged 74.6 ± 5.2 years) | Plasma levels of vitamin E (αTF, βTF, γTF, δTF, αT3, βT3, γT3, δT3) | 1 year follow-up | Low levels of vitamin E isomers (αTF, γTF, αT3, βT3, γT3, δT3) was detected in Alzheimer’s disease patients |
| Mangialasche et al. (2013) [285] | Population-based cohort study | Subjects with Alzheimer’s disease (n = 40) and MCI (n = 24) (aged 71.5 ± 3.8 years) | Baseline serum vitamin E | 8 years follow-up | High levels of TF and T3 were associated with reduced risk of cognitive impairment in older adults |
| Mangialasche et al. (2012) [287] | Cross-sectional study | Subjects with Alzheimer’s disease (n = 168; aged 77.4 ± 6.3 years) and MCI (n = 166; aged 75.8 ± 5.6 years) | Plasma vitamin E and markers of vitamin E damage (α-tocopherylquinone & 5-nitro-γTF) | - | Low levels of total TF, total T3 and total vitamin E in subjects with Alzheimer’s disease and MCI |
| In Vitro Studies | |||||
| Researcher (Year) | Cell Type | Treatment & Dose | Findings | ||
| Xu et al. (2017) [295] | Human fibroblasts and human dermal microvascular endothelial cells | MeT3α (10 nmol) | KIF26A: ↑, TRIM54: ↑, TOR2A: ↑, COX7A: ↑, MYH14: ↑, lanosterol synthase expression: ↓, VEGFA: ↑, PDGFB: ↑, basal and reserve mitochondrial capacity: ↑ | ||
| Arffah et al. (2009) [302] | Fibroblasts from hypertrophic scar (HSc). | TRF (0 to 0.156 mg/ml)—incubated for 24, 48 and 72 h | Cell growth: ↑ (at lower concentration, 0.0025 mg/ml to 0.02 mg/ml), fibroblast cell death: ↑ (at higher concentration, 0.078 mg/ml to 0.156 mg/ml), cell growth of hypertrophic scar fibroblast: ↓ | ||
| Mahirah et al. 2006 [303] | Fibroblast and keratinocyte cultures from hypertrophic scar | TRF—incubated for 24, 48 and 72 h | Cell growth: ↑ (at lower concentration), cell growth: ↓ (at higher concentrations) | ||
| In Vivo Studies | |||||
| Researcher (Year) | Animal Model | Treatment & Dose | Mode | Treatment Duration | Findings |
| Pierpaoli et al. (2017) [301] | BALB/c mice with excisional wounds inoculated with MRSA | Annatto T3 (12.9% γT3, 87.1% δT3) (100 mg/kg) | Oral | 8 days | Bacterial load: ↓, natural killer cell cytotoxicity: ↑, fibronectin type III: ↑, IL-24: ↑ |
| Elsy et al. (2017) [297] | Alloxan-induced diabetic albino rats with excisional wounds | d-δ-T3 (200 mg/kg) | Oral | 3 weeks | Early regeneration of epidermal and dermal components: ↑, blood sugar: ↓, TC: ↓, TG: ↓, LDL: ↓, VLDL: ↓, HDL: ↑, serum total protein content: ↑, CAT: ↑, antioxidant capacity: ↑ |
| Elsy et al. (2017) [298] | Alloxan-induced diabetic albino rats with incisional wounds | d-δ-TRF (200 mg/kg) | Oral | 3 weeks | Regeneration and reorganization of epidermal and dermal components: ↑ |
| Xu et al. (2017) [295] | Diabetic C57BL/KsJm/Leptdb (db/db) mice with excisional wounds | MeT3α (1 μmol) | Topical | 21 days | Wound area: ↓ |
| Musalmah et al. (2005) [299] | STZ-induced diabetic rats with excisional wounds | PVE (200 mg/kg) | Oral | 10 days | Wound area: ↓, total protein content: ↑, collagen content: ↑, GPx: ↑, SOD: ↑, MDA: ↓, |
| Nurlaily et al. (2011) [300] | Diabetic Sprague-Dawley male rats with excisional wounds | 0.5% TRF | Topical | Once daily for 10 days | Better wound contraction on 8th day post-treatment, total protein content: ↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Mohd Fahami, N.A.; Mohamed, I.N.; Shuid, A.N.; Mohd Saad, Q.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010259
Wong SK, Kamisah Y, Mohamed N, Muhammad N, Masbah N, Mohd Fahami NA, Mohamed IN, Shuid AN, Mohd Saad Q, Abdullah A, et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients. 2020; 12(1):259. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010259
Chicago/Turabian StyleWong, Sok Kuan, Yusof Kamisah, Norazlina Mohamed, Norliza Muhammad, Norliana Masbah, Nur Azlina Mohd Fahami, Isa Naina Mohamed, Ahmad Nazun Shuid, Qodriyah Mohd Saad, Azman Abdullah, and et al. 2020. "Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence" Nutrients 12, no. 1: 259. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010259