Magnesium Status and Stress: The Vicious Circle Concept Revisited
Abstract
:1. Introduction
2. Magnesium: Biological Role and Dietary Needs
2.1. Biological Role of Magnesium and Homeostasis
2.2. Food Sources, Current Recommended Intakes and Safety
2.3. Magnesium Deficiency: Causes and Health Consequences
3. Stress
3.1. Neurobiological Stress and Allostatic Load Model
3.2. Generalized Unsafety Theory of Stress (GUTS) Model
4. Evidence of the Impact of Stress on Magnesium Homeostasis
5. Evidence of the Impact of Magnesium Status on Stress Susceptibility
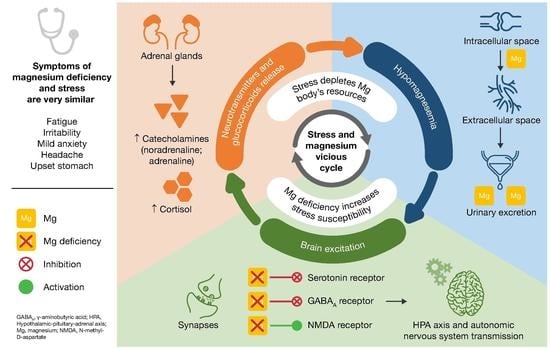
6. Proposed Model for the Vicious Circle of Stress and Magnesium Deficiency
- Magnesium and HPA. 5-HT transmission: Magnesium directly enhances the interaction between 5-HT and its membrane receptor, and it promotes the cellular transmission of the serotoninergic signal (Figure 2A) [90]. Additionally, magnesium is a cofactor of tryptophan hydroxylase, the enzyme involved in 5-HT synthesis [90]. Glutamatergic transmission: Magnesium inhibits the glutamate directly and indirectly by blocking the glutamate N-methyl-D-aspartate (NMDA) receptor and by enhancing its reuptake in the synaptic vesicles through stimulation of the sodium–potassium ATPase, respectively (Figure 2B) [42]. GABA transmission: A GABA-agonistic activity of magnesium has been observed, although the mechanism has not yet been elucidated, (Figure 2B) [42]. Cortisol: Magnesium indirectly reduces the release of ACTH by modulating the neurotransmission pathways, and therefore decreases cortisol levels in the body [42];
7. Magnesium Supplementation
8. Conclusions: Implications in Terms of Dietary Magnesium Needs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Chatterji, S.; Lee, S.; Ormel, J.; Üstün, T.B.; Wang, P.S. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) Surveys. Epidemiol. Psichiatr. Soc. 2009, 18, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Renal. Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, P.C.; Pham, P.M.; Pham, S.V.; Miller, J.M.; Pham, P.T. Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- American Psychological Association. Stress Effects on the Body. Available online: https://www.apa.org/helpcenter/stress (accessed on 30 June 2020).
- American Psychological Association. Understanding Chronic Stress. Available online: https://www.apa.org/helpcenter/understanding-chronic-stress.aspx (accessed on 30 June 2020).
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Galland, L. Magnesium, stress and neuropsychiatric disorders. Magnes. Trace Elem. 1991, 10, 287–301. [Google Scholar]
- Seelig, M.S. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review). J. Am. Coll. Nutr. 1994, 13, 429–446. [Google Scholar] [CrossRef]
- American Psychological Association. 2015 Stress in America. Available online: https://www.apa.org/news/press/releases/stress/2015/snapshot (accessed on 10 August 2020).
- Costello, R.; Wallace, T.C.; Rosanoff, A. Magnesium. Adv. Nutr. 2016, 7, 199–201. [Google Scholar] [CrossRef]
- Reddy, S.T.; Soman, S.S.; Yee, J. Magnesium Balance and Measurement. Adv. Chronic Kidney Dis. 2018, 25, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, C.; Gray-Scott, D.; Chen, J.J.; Meacham, S. What is next for the Dietary Reference Intakes for bone metabolism related nutrients beyond calcium: Phosphorus, magnesium, vitamin D, and fluoride? Crit Rev Food Sci. Nutr. 2009, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghabriel, M.N.; Vink, R. Magnesium transport across the blood-brain barriers. In Magnesium in the Central Nervous System; Nechifor, M., Vink, R., Eds.; The University of Adelaide Press: Adelaide, Australia, 2011; pp. 59–74. [Google Scholar] [CrossRef]
- Morris, M.E. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: Neurological symptoms. Magnes. Res. 1992, 5, 303–313. [Google Scholar] [PubMed]
- Chutkow, J.G. Uptake of magnesium into the brain of the rat. Exp. Neurol. 1978, 60, 592–602. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.A.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 159–175. [Google Scholar]
- Clarkson, E.M.; Warren, R.L.; McDonald, S.J.; de Wardener, H.E. The effect of a high intake of calcium on magnesium metabolism in normal subjects and patients with chronic renal failure. Clin. Sci. 1967, 32, 11–18. [Google Scholar]
- Norman, D.A.; Fordtran, J.S.; Brinkley, L.J.; Zerwekh, J.E.; Nicar, M.J.; Strowig, S.M.; Pak, C.Y. Jejunal and ileal adaptation to alterations in dietary calcium: Changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J. Clin. Investig. 1981, 67, 1599–1603. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S. The multifaceted and widespread pathology of magnesium deficiency. Med. Hypotheses 2001, 56, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Ayuk, J.; Gittoes, N.J. Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem. 2014, 51, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, L.M.; DM, N.F.; Gaydadzhieva, G.T.; Mazurkiewicz, O.M.; Leeson, H.; Wright, C.P. Magnesium in pregnancy. Nutr. Rev. 2016, 74, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, A. Hypomagnesaemia and pregnancy. Obstet. Med. 2018, 11, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Di Martino, M.; Pellegrino, P. Magnesium in the gynecological practice: A literature review. Magnes. Res. 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Seelig, M.S.; Preuss, H.G. Magnesium metabolism and perturbations in the elderly. Geriatr. Nephrol. Urol. 1994, 4, 101–111. [Google Scholar] [CrossRef]
- Lo Piano, F.; Corsonello, A.; Corica, F. Magnesium and elderly patient: The explored paths and the ones to be explored: A review. Magnes. Res. 2019, 32, 1–15. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002, 39, 209–213. [Google Scholar] [CrossRef]
- Viering, D.H.H.M.; de Baaij, J.H.F.; Walsh, S.B.; Kleta, R.; Bockenhauer, D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr. Nephrol. 2017, 32, 1123–1135. [Google Scholar] [CrossRef] [Green Version]
- Naderi, A.S.A.; Reilly, R.F. Hereditary etiologies of hypomagnesemia. Nat. Clin. Pract. Nephrol. 2008, 4, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar] [PubMed]
- Laires, M.J.; Monteiro, C. Exercise and Magnesium. In New Perspectives in Magnesium Research: Nutrition and Health; Nishizawa, Y., Morii, H., Durlach, J., Eds.; Springer: London, UK, 2007; pp. 173–185. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Welsh, R.C.; Haykowsky, M.J.; Taylor, D.A.; Humen, D.P. Biochemical changes as a result of prolonged strenuous exercise. Br. J. Sports Med. 2002, 36, 301. [Google Scholar] [CrossRef] [Green Version]
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients 2019, 11, 2335. [Google Scholar] [CrossRef] [Green Version]
- Murck, H. Magnesium and affective disorders. Nutr. Neurosci. 2002, 5, 375–389. [Google Scholar] [CrossRef]
- Cernak, I.; Savic, V.; Kotur, J.; Prokic, V.; Kuljic, B.; Grbovic, D.; Veljovic, M. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes. Res. 2000, 13, 29–36. [Google Scholar]
- Grases, G.; Pérez-Castelló, J.A.; Sanchis, P.; Casero, A.; Perelló, J.; Isern, B.; Rigo, E.; Grases, F. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes. Res. 2006, 19, 102–106. [Google Scholar]
- Classen, H.G. Systemic stress, magnesium status and cardiovascular damage. Magnesium 1986, 5, 105–110. [Google Scholar]
- United States Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee—Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; USDA Agricoltural Research Service: Washngton, DC, USA, 2015.
- Agence Nationale de Sécurité Sanitaire de L’alimentation de L’environnement et du Travail (ANSES). TANSES-CIQUAL French Food Composition Table, Version 2017; ANSES: Maisons-Alfort, France, 2017. [Google Scholar]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Ford, E.S.; Mokdad, A.H. Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winiarska-Mieczan, A.; Zaricka, E.; Kwiecień, M.; Kwiatkowska, K.; Baranowska-Wójcik, E.; Danek-Majewska, A. Can Cereal Products Be an Essential Source of Ca, Mg and K in the Deficient Diets of Poles? Biol. Trace Elem. Res. 2020, 195, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi-Boccia, G.; Aguzzi, A.; Cappelloni, M.; Di Lullo, G.; Lucarini, M. Total-diet study: Dietary intakes of macro elements and trace elements in Italy. Br. J. Nutr. 2003, 90, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. The Problematic Use of Dietary Reference Intakes to Assess Magnesium Status and Clinical Importance. Biol. Trace Elem. Res. 2019, 188, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Xun, P.; He, K.; Qin, L.Q. Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, R.; Apgar, B.J.; Wien, E.M. Apparent absorption and retention of Ca, Cu, Mg, Mn, and Zn from a diet containing bran. Am. J. Clin. Nutr. 1986, 43, 444–455. [Google Scholar] [CrossRef]
- Lakshmanan, F.L.; Rao, R.B.; Kim, W.W.; Kelsay, J.L. Magnesium intakes, balances, and blood levels of adults consuming self-selected diets. Am. J. Clin. Nutr. 1984, 40, 1380–1389. [Google Scholar] [CrossRef]
- Greger, J.L.; Baier, M.J. Effect of dietary aluminum on mineral metabolism of adult males. Dev. Med. Child Neurol. 1983, 38, 411–419. [Google Scholar] [CrossRef]
- Standing Commitee on the Scientific Evaluation of Dietary Reference Intakes Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Acedemies Press, Ed.; The National Academies Press: Washington, DC, USA, 1997; pp. 190–249. [Google Scholar]
- Jarosz, M. Nutritional Guidelines for the Polish Population; National Food and Nutrition Institute, Ed.; National Food and Nutrition Institute: Warsaw, Poland, 2017. [Google Scholar]
- Tutel’ian, V.A. Norms of physiological requirements in energy and nutrients in various groups of population in Russian Federation. Vopr. Pitan. 2009, 78, 4–15. [Google Scholar]
- Ministry of Health Labour and Welfare. Dietary Reference Intakes for Japanese; Japan Government Printing Office, Ed.; Ministry of Health Labour and Welfare: Tokyo, Japan, 2015. [Google Scholar]
- Società Italiana di Nutrizione Umana-SINU. LARN—Livelli di Assunzione di Riferimento per la Popolazione Italiana: MINERALI. Assunzione Raccomandata per la Popolazione (PRI in Grassetto) e Assunzione Adeguata (AI in Corsivo): Valori su Base Giornaliera. Available online: https://sinu.it/2019/07/09/minerali-assunzione-raccomandata-per-la-popolazione-pri-e-assunzione-adeguataai/ (accessed on 30 June 2020).
- Agence Nationale de Sécurité Sanitaire de L’alimentation de L’environnement et du Travail (ANSES). Avis de l’ANSES. In Actualisation des Repères du PNNS: Élaborationdes Références Nutritionnelles; ANSES, Ed.; ANSES: Maisons-Alfort, France, 2016. [Google Scholar]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Fulgoni, V.L., 3rd; Keast, D.R.; Dwyer, J.T. Dietary supplement use is associated with higher intakes of minerals from food sources. Am. J. Clin. Nutr. 2011, 94, 1376–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Public Health England. Official Statistics NDNS: Results from Years 7 and 8 (Combined). Results of the National Diet and Nutrition Survey (NDNS) Rolling Programme for 2014 to 2015 and 2015 to 2016; Public Health England: England, UK, 2018.
- Agence Nationale de Sécurité Sanitaire de L’alimentation de L’environnement et du Travail (ANSES). Avis de l’ANSES relatif à l’évaluation des apports en vitamines et minéraux issus de l’alimentation non enrichie, de l’alimentation enrichie et des compléments alimentaires dans la population Française: estimation des aApports usuels, des prévalences d’inadéquation et des risques de dépassement des limites de sécurité (Saisine n°2012-SA-0142); ANSES, Ed.; ANSES: Maisons-Alfort, France, 2015. [Google Scholar]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary Micronutrient and Mineral Intake in the Mediterranean Healthy Eating, Ageing, and Lifestyle (MEAL) Study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Health, Office of Dietary Supplements. Magnesium. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/#en1 (accessed on 10 August 2020).
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; The National Academies Press: Washington, DC, USA, 1997; p. 448. [Google Scholar] [CrossRef]
- Spätling, L.; Classen, H.G.; Külpmann, W.R.; Manz, F.; Rob, P.M.; Schimatschek, H.F.; Vierling, W.; Vormann, J.; Weigert, A.; Wink, K. Diagnosing magnesium deficiency. Current recommendations of the Society for Magnesium Research. Fortschr. Med. Orig. 2000, 118 (Suppl. 2), 49–53. [Google Scholar]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- Topf, J.M.; Murray, P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 4, 195–206. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, S194–S198. [Google Scholar] [CrossRef]
- Noah, L.; Pickering, G.; Mazur, A.; Dubray, C.; Hitier, S.; Dualé, C.; Pouteau, E. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: Secondary data from a randomised controlled trial. Magnes. Res. J. 2020, 33, 45–57. [Google Scholar]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Verwoert, G.C.; Hwang, S.J.; Glazer, N.L.; Smith, A.V.; van Rooij, F.J.; Ehret, G.B.; Boerwinkle, E.; Felix, J.F.; Leak, T.S.; et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet. 2010, 6, e1001045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshfegh, A.; Goldman, J.; Ahuja, J.; Rhodes, D.; LaComb, R. What We Eat in America, NHANES 2005–2006. Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamid D, Calcium, Phosphorus, and Magnesium; United States Department of Agriculture, Agricultural Research Service, Eds.; 2009. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0506/usual_nutrient_intake_vitD_ca_phos_mg_2005-06.pdf (accessed on 30 June 2020).
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Thomas, D. The mineral depletion of foods available to us as a nation (1940–2002)—A Review of the 6th Edition of McCance and Widdowson. Nutr. Health 2007, 19, 21–55. [Google Scholar] [CrossRef]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The Role of Nutrients in Protecting Mitochondrial Function and Neurotransmitter Signaling: Implications for the Treatment of Depression, PTSD, and Suicidal Behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [Green Version]
- Fromm, L.; Heath, D.L.; Vink, R.; Nimmo, A.J. Magnesium Attenuates Post-Traumatic Depression/Anxiety Following Diffuse Traumatic Brain Injury in Rats. J. Am. Coll. Nutr. 2004, 23, 529S–533S. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M. Impaired Magnesium Status and Depression. In Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy; Preedy, V.R., Patel, V.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1861–1872. [Google Scholar] [CrossRef]
- Cuciureanu, M.; Vink, R. Magnesium and stress. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.S.; Bond, M.J.; Hecker, J.R. Environmental stress, psychological stress and allostatic load. Psychol. Health Med. 2007, 12, 18–30. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad Sci 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- Carrasco, G.A.; Van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharm. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Lanfumey, L.; Mongeau, R.; Cohen-Salmon, C.; Hamon, M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008, 32, 1174–1184. [Google Scholar] [CrossRef]
- Herman, J.P.; Mueller, N.K.; Figueiredo, H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann. N. Y. Acad. Sci. 2004, 1018, 35–45. [Google Scholar] [CrossRef]
- Nakamura, J.; Yakata, M. Two-cycle liquid-chromatographic quantitation of cortisol in urine. Clin. Chem. 1982, 28, 1497–1500. [Google Scholar] [CrossRef]
- Singh, A.; Petrides, J.S.; Gold, P.W.; Chrousos, G.P.; Deuster, P.A. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J. Clin. Endocrinol. Metab. 1999, 84, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, E.; Perry, S.W.; Licinio, J.; Wong, M.-L.; Dremencov, E.; Zavjalov, E.L.; Shevelev, O.B.; Khotskin, N.V.; Koncevaya, G.V.; Khotshkina, A.S.; et al. From Allostatic Load to Allostatic State—An Endogenous Sympathetic Strategy to Deal With Chronic Anxiety and Stress? Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Assis, G.G.; Gasanov, E.V. BDNF and Cortisol integrative system—Plasticity vs. degeneration: Implications of the Val66Met polymorphism. Front. Neuroendocrinol. 2019, 55. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef]
- Wallingford, J.K.; Deurveilher, S.; Currie, R.W.; Fawcett, J.P.; Semba, K. Increases in mature brain-derived neurotrophic factor protein in the frontal cortex and basal forebrain during chronic sleep restriction in rats: Possible role in initiating allostatic adaptation. Neuroscience 2014, 277, 174–183. [Google Scholar] [CrossRef]
- Yuluğ, B.; Ozan, E.; Gönül, A.S.; Kilic, E. Brain-derived neurotrophic factor, stress and depression: A minireview. Brain Res. Bull. 2009, 78, 267–269. [Google Scholar] [CrossRef]
- Colaianna, M.; Schiavone, S.; Zotti, M.; Tucci, P.; Morgese, M.G.; Bäckdahl, L.; Holmdahl, R.; Krause, K.-H.; Cuomo, V.; Trabace, L. Neuroendocrine profile in a rat model of psychosocial stress: Relation to oxidative stress. Antioxid. Redox. Signal 2013, 18, 1385–1399. [Google Scholar] [CrossRef] [Green Version]
- Kapczinski, F.; Vieta, E.; Andreazza, A.C.; Frey, B.N.; Gomes, F.A.; Tramontina, J.; Kauer-Sant’anna, M.; Grassi-Oliveira, R.; Post, R.M. Allostatic load in bipolar disorder: Implications for pathophysiology and treatment. Neurosci. Biobehav. Rev. 2008, 32, 675–692. [Google Scholar] [CrossRef]
- Danhof-Pont, M.B.; van Veen, T.; Zitman, F.G. Biomarkers in burnout: A systematic review. J. Psychosom. Res. 2011, 70, 505–524. [Google Scholar] [CrossRef]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the NMDA receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Vink, R. Magnesium in the CNS: Recent advances and developments. Magnes. Res. 2016, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. Biomed. Taipei 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Brosschot, J.F.; Verkuil, B.; Thayer, J.F. The default response to uncertainty and the importance of perceived safety in anxiety and stress: An evolution-theoretical perspective. J. Anxiety. Disord. 2016, 41, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Somjen, G.G.; Baskerville, E.N. Effect of Excess Magnesium on Vagal Inhibition and Acetylcholine Sensitivity of the Mammalian Heart in situ and in vitro. Nature 1968, 217, 679–680. [Google Scholar] [CrossRef]
- Toda, N.; West, T. Interaction between Na, Ca, Mg, and vagal stimulation in the S-A node of the rabbit. Am. J. Physiol. Leg. Content 1967, 212, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Murasato, Y.; Harada, Y.; Ikeda, M.; Nakashima, Y.; Hayashida, Y. Effect of Magnesium Deficiency on Autonomic Circulatory Regulation in Conscious Rats. Hypertension 1999, 34, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Brosschot, J.F.; Verkuil, B.; Thayer, J.F. Generalized Unsafety Theory of Stress: Unsafe Environments and Conditions, and the Default Stress Response. Int. J. Environ. Res. Public Health 2018, 15, 464. [Google Scholar] [CrossRef] [Green Version]
- Stoian, M.; Stoica, V. The role of distubances of phosphate metabolism in metabolic syndrome. Maedica Buchar. 2014, 9, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Brosschot, J.F. Ever at the ready for events that never happen. Eur. J. Psychotraumatol. 2017, 8, 1309934. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, A.L. The Effects of Psychological and Environmental Stress on Micronutrient Concentrations in the Body: A Review of the Evidence. Adv. Nutr. 2019, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Classen, H.G.; Marquardt, P.; Späth, M.; Schumacher, K.A. Hypermagnesemia Following Exposure to Acute Stress. Pharmacology 1971, 5, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ising, H.; Handrock, M.; Günther, T.; Fischer, R.; Dombrowski, M. Increased noise trauma in guinea pigs through magnesium deficiency. Arch. Oto Rhino Laryngol. 1982, 236, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Joachims, Z.; Babisch, W.; Ising, H.; Günther, T.; Handrock, M. Dependence of noise-induced hearing loss upon perilymph magnesium concentration. J. Acoust. Soc. Am. 1983, 74, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ising, H. Interaction of noise-induced stress and Mg decrease. Artery 1981, 9, 205–211. [Google Scholar]
- Ando, I.; Karasawa, K.; Yokota, S.; Shioya, T.; Matsuda, H.; Tanaka, A. Analysis of serum magnesium ions in dogs exposed to external stress: A pilot study. Open Vet. J. 2017, 7, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, F.; Haleem, D.J.; Haleem, M.A. Effects of repeated restraint stress on serum electrolytes in ethanol-treated and water-treated rats. Pak. J. Pharm. Sci. 2007, 20, 51–55. [Google Scholar]
- Heroux, O.; Peter, D.; Heggtveit, A. Long-term Effect of Suboptimal Dietary Magnesium on Magnesium and Calcium Contents of Organs, on Cold Tolerance and on Lifespan, and its Pathological Consequences in Rats. J. Nutr. 1977, 107, 1640–1652. [Google Scholar] [CrossRef]
- Terashima, Y.; Tucker, R.E.; Deetz, L.E.; Degregorio, R.M.; Muntifering, R.B.; Mitchell, G.E., Jr. Plasma Magnesium Levels as Influenced by Cold Exposure in Fed or Fasted Sheep. J. Nutr. 1982, 112, 1914–1920. [Google Scholar] [CrossRef] [Green Version]
- Whyte, K.F.; Addis, G.J.; Whitesmith, R.; Reid, J.L. Adrenergic control of plasma magnesium in man. Clin. Sci. Lond. 1987, 72, 135–138. [Google Scholar] [CrossRef]
- Tanabe, K.; Osada, N.; Suzuki, N.; Nakayama, M.; Yokoyama, Y.; Yamamoto, A.; Oya, M.; Murabayashi, T.; Yamamoto, M.; Omiya, K.; et al. Erythrocyte magnesium and prostaglandin dynamics in chronic sleep deprivation. Clin. Cardiol. 1997, 20, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Takase, B.; Akima, T.; Uehata, A.; Ohsuzu, F.; Kurita, A. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin. Cardiol. 2004, 27, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mocci, F.; Canalis, P.; Tomasi, P.A.; Casu, F.; Pettinato, S. The effect of noise on serum and urinary magnesium and catecholamines in humans. Occup. Med. 2001, 51, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ising, H.; Dienel, D.; Günther, T.; Markert, B. Health effects of traffic noise. Int. Arch. Occup. Environ. Health 1980, 47, 179–190. [Google Scholar] [CrossRef]
- Joborn, H.; Akerström, G.; Ljunghall, S. Effects of exogenous catecholamines and exercise on plasma magnesium concentrations. Clin. Endocrinol. 1985, 23, 219–226. [Google Scholar] [CrossRef]
- Caddell, J.; Kupiecki, R.; Proxmire, D.; Satoh, P.; Hutchinson, B. Plasma Catecholamines in Acute Magnesium Deficiency in Weanling Rats. JN 1986, 116, 1896–1901. [Google Scholar] [CrossRef]
- Amyard, N.; Leyris, A.; Monier, C.; Francès, H.; Boulu, R.G.; Henrotte, J.G. Brain catecholamines, serotonin and their metabolites in mice selected for low (MGL) and high (MGH) blood magnesium levels. Magnes. Res. 1995, 8, 5–9. [Google Scholar]
- Henrotte, J.G.; Franck, G.; Santarromana, M.; Frances, H.; Mouton, D.; Motta, R. Mice selected for low and high blood magnesium levels: A new model for stress studies. Physiol. Behav. 1997, 61, 653–658. [Google Scholar] [CrossRef]
- Henrotte, J.G.; Aymard, N.; Allix, M.; Boulu, R.G. Effect of pyridoxine and magnesium on stress-induced gastric ulcers in mice selected for low or high blood magnesium levels. Ann. Nutr. Metab. 1995, 39, 285–290. [Google Scholar] [CrossRef]
- Sartori, S.B.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 62, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Spasov, A.A.; Iezhitsa, I.N.; Kharitonova, M.V.; Kravchenko, M.S. Depression-like and anxiety-related behaviour of rats fed with magnesium-deficient diet. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni IP Pavlova 2008, 58, 476–485. [Google Scholar]
- Iezhitsa, I.N.; Spasov, A.A.; Kharitonova, M.V.; Kravchenko, M.S. Effect of magnesium chloride on psychomotor activity, emotional status, and acute behavioural responses to clonidine, d-amphetamine, arecoline, nicotine, apomorphine, and L-5-hydroxytryptophan. Nutr. Neurosci. 2011, 14, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Akarachkova, E. The role of magnesium deficiency in the formation of clinical manifestation of stress in women. Probl. Women Health 2013, 8, 57. [Google Scholar]
- Nielsen, F.H.; Johnson, L.K.; Zeng, H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes. Res. 2010, 23, 158–168. [Google Scholar] [CrossRef]
- Hermes Sales, C.; Azevedo Nascimento, D.; Queiroz Medeiros, A.C.; Costa Lima, K.; Campos Pedrosa, L.F.; Colli, C. There is chronic latent magnesium deficiency in apparently healthy university students. Nutr. Hosp. 2014, 30, 200–204. [Google Scholar] [CrossRef]
- Eby, G.A.; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef]
- Forsyth, A.K.; Williams, P.G.; Deane, F.P. Nutrition status of primary care patients with depression and anxiety. Aust. J. Prim. Health 2012, 18, 172–176. [Google Scholar] [CrossRef]
- Abumaria, N.; Yin, B.; Zhang, L.; Li, X.Y.; Chen, T.; Descalzi, G.; Zhao, L.; Ahn, M.; Luo, L.; Ran, C.; et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J. Neurosci. 2011, 31, 14871–14881. [Google Scholar] [CrossRef]
- Pochwat, B.; Sowa-Kucma, M.; Kotarska, K.; Misztak, P.; Nowak, G.; Szewczyk, B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology 2015, 232, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg(2+) in Regulation of Cellular and Mitochondrial Functions. Oxid Med. Cell Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zogović, D.; Pesić, V.; Dmitrasinović, G.; Dajak, M.; Plećas, B.; Batinić, B.; Popović, D.; Ignjatović, S. Pituitary-gonadal, pituitary-adrenocortical hormonal and IL-6 levels following long-term magnesium supplementation in male students. J. Med. Biochem. 2014, 33, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Wienecke, E.; Nolden, C. Long-term HRV analysis shows stress reduction by magnesium intake. MMW Fortschr. Med. 2016, 158, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, G.A.; Eby, K.L. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses 2006, 67, 362–370. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress-A Systematic Review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef] [Green Version]



| Most Frequently Reported Symptoms of Stress [6,7] | Symptoms of Magnesium Deficiency [5,8] |
|---|---|
| Fatigue | Tiredness |
| Irritability or anger | Irritability |
| Feeling nervous | Mild anxiety/nervousness |
| Lack of energy | Muscle weakness |
| Upset stomach | Gastrointestinal spasms |
| Muscle tension | Muscle cramps |
| Headache | Headache |
| Sadness/depression | Mild sleep disorders |
| Chest pain/hyperventilation | Nausea/vomiting |
| Diet related |
| Inadequate magnesium intake [5,13,26] |
| High protein diet [5,25] |
| High sodium diet [5,25] |
| High calcium diet [5,23,24,25] |
| High caffeine intake [5] |
| Alcohol dependence [5,13,26] |
| Lifestyle |
| Sports [25,38,39,40] |
| Sleep quality and quantity [41,42] |
| Chronic stress [43,44] |
| Pharmacological related |
| Diuretics, e.g., furosemide [13,26,27] |
| Proton-pump inhibitors, e.g., omeprazole [13,27] |
| Cisplatin [13,26,27] |
| Antibiotics, e.g., gentamicin [13,27] |
| Physiological conditions |
| Pregnancy [28,29] |
| Ageing [31,32] |
| Menopause [30] |
| Pathological conditions |
| Genetic disorders [8,35,36] |
| Type 2 diabetes mellitus [4,5] |
| Gastrointestinal disorders [5,26] |
| Kidney failure [5,33] |
| Cardiovascular diseases [5,45] |
| Metabolic syndrome [5,34] |
| Osteoporosis [15,25,33] |
| Country | Magnesium, mg/day | |
|---|---|---|
| Men | Women | |
| Italy [62] | 240 | 240 |
| Russia [60] | 300 | 300 |
| Japan [61] | 320–340 | 220–230 |
| Poland [59] | 400–420 | 310–320 |
| USA and Canada [58] | 400–420 | 310–320 |
| France [63] | 420 | 360 |
| Evidence of the Impact of Stress on Magnesium Homeostasis | |||
|---|---|---|---|
| Population Tested | Stress Stimulus | Impact on Magnesium | |
| Pre-clinical | Cats (N = 30) | Withdrawal of blood; infusion of catecholamines; potassium poisoning | ↑Blood Mg [122] |
| Guinea pigs (41) | Noise | ↑Serum Mg, ↓Erythrocytes Mg [123] | |
| Rats (88) | Noise | ↑Serum Mg, ↓Erythrocytes Mg [124] | |
| Rats | Noise | ↓Serum Mg, ↓Erythrocytes Mg | |
| Dogs | Physical exercise, temperature | ↓Serum Mg [126] | |
| Rats | Ethanol/Restraint stress | ↓Serum Mg [127] | |
| Rats | Cold | ↓Tissue content of Mg [129] | |
| Sheep | Dietary Mg restriction, cold | ↓Plasma Mg [129] | |
| Clinical | Adults (N = 8) | Adrenaline infusion | ↓Plasma Mg [130] |
| Young adults (N = 35) | Chronic or sub-chronic psychological stress | ↓Plasma Mg [43] | |
| Healthy men (N = 16) | Chronic sleep deprivation | ↓Erythrocyte Mg [131] | |
| Young adults (N = 35) | University exams | ↑Urinary Mg [44] | |
| Young adults (N = 30) | University exams | ↓Erythrocyte Mg [132] | |
| Young adults (N = 25) | Noise | ↑Urinary Mg ↑Serum Mg [133] | |
| Healthy men (56) | Noise | ↑Serum Mg, ↓Erythrocytes Mg; ↑Urinary Mg [134] | |
| Healthy men | Short- and long-term physical exercise | ↑Plasma Mg [135] | |
| Evidence of the Impact of Magnesium Status on Stress Susceptibility | ||||
|---|---|---|---|---|
| Population Tested | Mg Status | Stress Stimulus | Impact on Stress Mediator/Stress | |
| Pre-clinical | Rats (N = 84) | Mg-deficient | Noise | ↑Catecholamines (NA, adrenaline, dopamine) [136] |
| Mice (N = 120) | Mg-deficient | Genetic selection | ↑NA [137] | |
| Mice (N = 80) | Mg-deficient | Genetic selection; forced swimming test; four-plate test | ↑NA [138] | |
| Mice (N = 100) | Mg-deficient | Genetic selection; immobilization test | ↑Gastric ulcers [139] | |
| Mice (N = 20/test) | Dietary Mg restriction | Hyperthermia; open field test; light/dark test; hyponeophagia test | ↑CRH; ↑ACHT [140] | |
| Mice | Mg-deficient | Light/dark test | Depression-like behavior [42,140] | |
| Rats | Dietary Mg restriction | Forced swimming test | Depression-like behavior [142,143] | |
| Rats | Dietary Mg restriction | Open field test | Stress/anxiety [142,143] | |
| Clinical | Women (N = 100) | Mg-deficient | - | Chronic emotional stress; irritability; fatigue; sleep disturbance; headache a [144] |
| Adults (N = 264) | Mg-deficient | - | Severe stress [78,145,146] | |
| Adults (N = 100) | Mg-deficient | Poor sleep quality | ↑CRP [145] | |
| Adults (N = 109) | Mg-deficient | - | Depression/anxiety [148] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123672
Pickering G, Mazur A, Trousselard M, Bienkowski P, Yaltsewa N, Amessou M, Noah L, Pouteau E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients. 2020; 12(12):3672. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123672
Chicago/Turabian StylePickering, Gisèle, André Mazur, Marion Trousselard, Przemyslaw Bienkowski, Natalia Yaltsewa, Mohamed Amessou, Lionel Noah, and Etienne Pouteau. 2020. "Magnesium Status and Stress: The Vicious Circle Concept Revisited" Nutrients 12, no. 12: 3672. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123672