Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health
Abstract
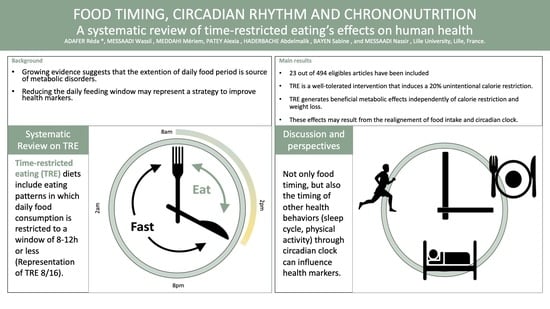
:1. Introduction
2. Method
2.1. Collection and Selection of Items
2.2. Data Analysis and Extraction
2.3. Level of Evidence Classification
3. Results and Discussion
3.1. Selected Articles and Characteristics
3.2. Types of Time-Restricted Eating: Clarification of Terms
3.3. Adherence to TRE and Effect on Calorie Consumption
3.4. Metabolic Effects of TRE
3.5. TRE and the Circadian Clock
3.6. Other Effects of TRE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zöllner, N. The relevance of diet for civilization diseases, especially atherosclerosis. Wien. Med. Wochenschr. Suppl. 1990, 106, 11–12. [Google Scholar]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Kant, A.K. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol. Behav. 2018, 193, 270–278. [Google Scholar] [CrossRef]
- Gupta, N.J.; Kumar, V.; Panda, S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 2017, 12, e0172852. [Google Scholar] [CrossRef]
- Marinac, C.R.; Nelson, S.H.; Breen, C.I.; Hartman, S.J.; Natarajan, L.; Pierce, J.P.; Flatt, S.W.; Sears, D.D.; Patterson, R.E. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol. 2016, 2, 1049–1055. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.-C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Marinac, C.R.; Sears, D.D.; Natarajan, L.; Gallo, L.C.; Breen, C.I.; Patterson, R.E. Frequency and Circadian Timing of Eating May Influence Biomarkers of Inflammation and Insulin Resistance Associated with Breast Cancer Risk. PLoS ONE 2015, 10, e0136240. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.E.; Laughlin, G.A.; Lacroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs. daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes. 2014, 39, 727–733. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.; Gill, S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melkani, G.C.; Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 2017, 595, 3691–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC5139140/ (accessed on 18 May 2020). [CrossRef] [Green Version]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guide HAS: Niveau de Preuve et Gradation des Recommandations de Bonne Pratique. Rédaction Médicale et Scientifique. Available online: https://www.redactionmedicale.fr/2013/06/mis-en-ligne-le-14-juin-2013-sur-le-site-de-la-has-ce-guide-est-bienvenu-mais-sadresse-aux-sp%C3%A9cialistes-du-sujet-sil-en-ex.html (accessed on 18 May 2020).
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7, E22. [Google Scholar] [CrossRef] [Green Version]
- Jamshed, H.; Beyl, R.; Manna, D.; Yang, E.; Ravussin, E.; Peterson, C. Early time-restricted feeding improves 24-h glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.T.; LeSarge, J.C.; Lemon, P.W. Time-Restricted Eating In Women—A Pilot Study. West. Undergrad. Res. J. Health Nat. Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [Green Version]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation but Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Burgess, H.J.; Varady, K.A. Effect of 8-h time-restricted feeding on sleep quality and duration in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-h time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Varady, K.A. Safety of 8-h time restricted feeding in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 107–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition 2018, 51–52, 29–37. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Sypniewski, C.; Bensadon, B.; McLaren, C.; Donahoo, W.T.; Sibille, K.T.; Anton, S.D. Determinants of Adherence in Time-Restricted Feeding in Older Adults: Lessons from a Pilot Study. Nutrients 2020, 12, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre–Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, M.J.; Manoogian, E.N.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Carter, M.C.; Burley, V.J.; Nykjaer, C.; Cade, J.E. Adherence to a Smartphone Application for Weight Loss Compared to Website and Paper Diary: Pilot Randomized Controlled Trial. J. Med. Internet Res. 2013, 15, e32. [Google Scholar] [CrossRef]
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and Real-World Settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Middleton, K.R.; Anton, S.D.; Perri, M.G. Long-Term Adherence to Health Behavior Change. Am. J. Lifestyle Med. 2013, 7, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Miguet, M.; Masurier, J.; Chaput, J.-P.; Pereira, B.; Lambert, C.; Dâmaso, A.; Courteix, D.; Duclos, M.; Boirie, Y.; Thivel, D. Cognitive restriction accentuates the increased energy intake response to a 10-month multidisciplinary weight loss program in adolescents with obesity. Appetite 2019, 134, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.; Bhapkar, M.; Das, S.; Galan, K.; Martin, C.; McAdams, L.; Pieper, C.; Redman, L.; Roberts, S.; Stein, R.; et al. Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) Screening and Recruitment: Methods and Results. Contemp. Clin. Trials 2013, 34, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Azevedo, F.R.; Caramelli, B. Effects of intermittent fasting on metabolism in men. Rev. Assoc. Med. Bras. 2013, 59, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Heran, B.S.; Wong, M.M.; Heran, I.K.; Wright, J.M. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst. Rev. 2008, 2008, CD003823. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [Green Version]
- Gulcelik, N.E.; Halil, M.; Ariogul, S.; Usman, A. Adipocytokines and aging: Adiponectin and leptin. Minerva Endocrinol. 2013, 38, 203–210. [Google Scholar]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, L.; Li, X.; Li, X.; Sun, G.; Yuan, X.; Lei, L.; Liu, J.; Yin, L.; Deng, Q.; et al. Adiponectin activates the AMPK signaling pathway to regulate lipid metabolism in bovine hepatocytes. J. Steroid Biochem. Mol. Biol. 2013, 138, 445–454. [Google Scholar] [CrossRef]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin Activates AMP-activated Protein Kinase in Muscle Cells via APPL1/LKB1-dependent and Phospholipase C/Ca2+/Ca2+/Calmodulin-dependent Protein Kinase Kinase-dependent Pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashiura, K.; Ura, N.; Ohata, J.-I.; Togashi, N.; Takagi, S.; Saitoh, S.; Murakami, H.; Takagawa, Y.; Shimamoto, K. Correlations of adiponectin level with insulin resistance and atherosclerosis in Japanese male populations. Clin. Endocrinol. 2004, 61, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation123. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol Res. Health 2001, 25, 85–93. [Google Scholar]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian Disruption and Metabolic Disease: Findings from Animal Models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Kessler, K.; Pivovarova-Ramich, O. Meal Timing, Aging, and Metabolic Health. Int. J. Mol. Sci. 2019, 20, 1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunderram, J.; Sofou, S.; Kamisoglu, K.; Karantza, V.; Androulakis, I.P. Time-restricted feeding and the realignment of biological rhythms: Translational opportunities and challenges. J. Transl. Med. 2014, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G.K. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Baden, M.Y.; Yamada, Y.; Takahi, Y.; Obata, Y.; Saisho, K.; Tamba, S.; Yamamoto, K.; Umeda, M.; Furubayashi, A.; Tsukamoto, Y.; et al. Association of adiponectin with blood pressure in healthy people. Clin. Endocrinol. 2013, 78, 226–231. [Google Scholar] [CrossRef]
- Hashinaga, T.; Wada, N.; Otabe, S.; Yuan, X.; Kurita, Y.; Kakino, S.; Tanaka, K.; Sato, T.; Kojima, M.; Ohki, T.; et al. Modulation by adiponectin of circadian clock rhythmicity in model mice for metabolic syndrome. Endocr. J. 2012, 60, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; DeSouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Barger, L.K.; Wright, K.P.; Hughes, R.J.; Czeisler, C.A. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R1077–R1084. [Google Scholar] [CrossRef] [Green Version]
- Guidoux, R.; Duclos, M.; Fleury, G.; Lacomme, P.; Lamaudière, N.; Manenq, P.-H.; Paris, L.; Ren, L.; Rousset, S. A smartphone-driven methodology for estimating physical activities and energy expenditure in free living conditions. J. Biomed. Inform. 2014, 52, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Kacimi, S.; Ref’at, A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-H.; Wu, W.; Kang, S.S.; Liu, X.; Wu, Z.; Peng, J.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Liu, X.; et al. BDNF inhibits neurodegenerative disease-associated asparaginyl endopeptidase activity via phosphorylation by AKT. JCI Insight 2018, 3, e99007. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]

| Study [Ref] | Level of Evidence | Population | Protocol | Energy Balance | Metabolic Effect | Other Results |
|---|---|---|---|---|---|---|
| R1 Antoni et al. 2018 [27] | Low | n = 13 Healthy adults, 29–57 years. | ↓ Food window of 3 h 10-week non-randomized controlled trial. | ↓ daily energy intake | ↓ body 1.9% fat mass index ↓ fasting blood glucose | Only 19% withdrawal including one lost to follow-up |
| R2 Jamshed et al. 2019 [28] | Medium | n = 11 Healthy adults, 32 ± 7 years. | Early TRE 6/18 4-day randomized controlled iso-caloric crossover trial with 3.5 to 5 weeks of wash-out | No difference in calorie intake (iso-caloric) | ↓ 24-h glucose and hyperglycemic excursion ↓ insulin resistance ↑ total cholesterol, LDLc, HDLc | ↑ BDNF ↓ IGF1 Modification of genes expressions involved in circadian rhythm, longevity, autophagy |
| R3 Smith et al. 2017 [29] | Low | n = 20 Healthy women, 21.3 ± 1.2 years. | Delayed TRE 8/16 4-week single-arm trial |  | ↓ body mass of 0.6 ± 1 kg ↓ body fat in participants that strength trained (>3 day/week) | |
| R4 Sutton et al. 2018 [30] | Medium | n = 8 Pre-diabetic overweighted men, 59 ± 9 years. | Early TRE 6/18 5-week controlled, randomized, isocaloric crossover trial with 7 weeks of wash-out | No difference in calorie intake (iso-caloric) | ↓ insulin (fasting, mean and peak) ↑ insulin sensitivity ↓ insulin resistance ↑ triglycerides ↓ blood pressure | ↓ desire to eat ↓ 8-isoprostane |
| R5 Ravussin et al. 2019 [31] | Medium | n = 11 Healthy adults, 32 years. | Early TRE 6/18 4-day controlled, randomized, iso-caloric crossover trial with 3.5 to 5 weeks of wash-out | No difference in calorie intake (standardized meals) Energy expenditure unchanged |  | ↓ several aspects of hunger ↓ in morning ghrelin, leptin and GLP-1 ↓ average ghrelin ↑ in evening PYY (satiety) |
| R6 Gabel et al. 2019 [32] | Low | n = 23 Obese, 50 ± 2 years. | TRE 8/16 12-week single-arm trial. | Physical activity unchanged. No measure of calorie intake | ↓ 4% weight ↓ 5% fat mass | 80% mean adherence. |
| R7 and 9 Gabel et al. 2018, 2014 [33,34] | Low | n = 23 Obese, 50 ± 2 years. | Delayed TRE 8/16 12-week, non-randomized controlled trial with matched historical group. | ↓ of 350 kcal/day Physical activity unchanged. | ↓ 2.6% of relative weight ↓ of relative BMI ↓ systolic blood pressure of 7 ± 2 mmHg | 74% adherence rate. No one in the TRE group reported dropping out due to issues with the diet. |
| R8 Moro et al. 2016 [35] | Medium | n = 34 Adults who strength train, 29.21 ± 3.8 years. | TRE 8/16 + RT 8-week randomized controlled trial. TRE + RT vs. RT | No difference in calorie intake between groups No difference in physical activity during training sessions | ↓ fat mass ↓ blood glucose levels ↓ insulin resistance ↓ triglycerides | ↓ TNF-α ↓ IL-1 β ↓ IGF 1 ↑ adiponectin ↓ respiratory ratio (lipid oxidation) Conservation of muscular mass and strength |
| R10 Anton et al. 2019 [36] | Low | n = 10 Overweighted elderly adults, 77.1 years. | TRE 8/16 4-week single-arm trial. |  | Mean weight ↓ of 2.6 kg | ↑ walking speed Improvement in mental and physical function. 84% mean adherence. |
| R11 Hutchison et al. 2019 [37] | Medium | n = 15 Pre-diabetic men, 55 ± 3 years. | dTRE 9/15 vs. eTRE 1-week cross-over, randomized trial with 2 weeks of wash-out | No difference in physical activity No measure of calorie intake | ↓ glucose AUC and mean fasting glucose in eTRE ↓ triglycerides in two groups | No effect of TRF on perceived hunger, fullness, or desire to eat. |
| R12 Tinsley et al. 2016 [38] | Medium | n = 18 Adults who strength-train, 22 ± 2.4 years. | TRE 4/20 + RT vs. RT alone. 8-week randomized controlled trial. | ↓ of 650 kcal/day between fasting days and non-fasting days ↓ weekly calorie intake | No significant change in weight and fat mass | Conservation of lean mass, muscular volume and muscular strength. 95% mean adherence. |
| R13 Gasmi et al. 2017 [39] | Medium | n = 40 20 y (n = 40) vs. 50 years (n = 20) | TRE 12/12: TRE 50 years + 20 years Control 50 years + 20 years 12-week randomized, controlled trial. | No difference in calorie intake. | No change in body composition and muscular function | ↓ immuno-senescence |
| R14 Tinsley et al. 2019 [40] | Medium | n = 40 Women who strength-train 18–30 years. | Delayed TRE 8/16 8-week randomized controlled trial. -RT + placebo -TRE + RT + placebo -TRE + RT + HMB | ↑ calorie intake from 20 to 200 kcal/day No difference in physical activity and REE | ↓ fat mass of 4%–7% in per protocol analysis for the 2 TRE groups | No side effects in 90% of participants at the end of the protocol |
| R15 Gill et al. 2015 [41] | Low | n= 8 Obese adults, 18 years. | TRE 10/14 every day, 3-week single-arm trial. Smartphone-based assessment of caloric quantity and timing intake | ↓ calorie intake of 20% | ↓ weight by 4% ↓ BMI by 1.15 kg/m2 | ↑ sleep quality ↓ hunger |
| R16 Lee et al. 2020 [42] | Low | n = 10 Overweight sedentary elderly adults, 77.1 years. | TRE 8/16 every day with self-selection of eating window. 4-week single-arm trial. |  |  | Mean adherence of 84%. |
| R17 Kesztyüs et al. 2019 [43] | Low | n = 40 Abdominally obese, 49.1 ± 12.4 years. | TRE 8/16 every day with self-selection of the food intake period 12-week single-arm trial. |  | ↓ weight of 1.7 ± 2.5 kg ↓ BMI of 0.6 ± 0.9 kg/m2 ↓ WC −5.3 ± 3.2 cm ↓ HbAc1 by 1.4 ± 3.5 mmol/mol | Mean adherence of 86 ± 15% |
| R18 Wilkinson et al. 2020 [44] | Low | n = 19 Adults with MetS 59 ± 11 years. | TRE 10/14 every day with self-selection of the food intake period. 12-week single-arm trial. | ↓ by 8.62% ± 14.47%. No difference in physical activity. | ↓ body weight (−3%) ↓ BMI (−3%) ↓ body fat (−3%) ↓ visceral fat rating (−3%) ↓ WC-4.46 ± 6.72 cm ↓ total cholesterol ↓ LDLc, ↓ non-HDLc ↓ systolic and diastolic BP | Mean adherence of 85 ± 12%. 63.2% participants were somehow engaged in TRE at 16 ± 4 months. ↑ in sleep duration by 12.45 min. ↑ in sleep duration and efficiency in 84% of participants. |
| R19 McAllister et al. 2019 [45] | Medium | n = 22 Physically active men, 22 ± 2.5 years. | TRE 8/16 every day ad libitum vs. Isocaloric (↓ 300 kcal from baseline). 4-week randomized controlled trial. | No difference in calorie intake | ↓ body mass in both groups ↓ body fat mass in both groups ↓ systolic BP in both groups ↑ HDLc in both groups | ↑ adiponectin in both groups. Improvement in subjective outcomes (alertness, energy, focus, mood) in ad libitum. |
| R20 Chow et al. 2020 [46] | Low | n = 20 Overweight adults with prolonged eating window (15.4 ± 0.9 h/day). 45.5 ± 12 years. | TRE 8/16 ad libitum every day. 12-week controlled non-randomized trial. TRE 8/16 group vs. non-TRE group. | No difference in physical activity. No measure of calorie intake. | (1) vs. non-TRE group ↓ body weight ↓ lean mass ↓ visceral fat (2) vs. preintervention measures ↓ body weight ↓ fat mass ↓ lean mass ↓ visceral fat | ↓ of eating window in TRE group (9.9 ± 2 h) compared with non-TRE group. Adherence in TRE: 83.1% Correlation between restriction of eating window with fat and visceral masses loss |
| R21 Parr et al. 2020 [47] | Medium | n = 11 Overweight/obese and sedentary men. 38 ± 5 years. | TRE 8/16 every day vs. non-TRE (15 h/day). 5-day randomized crossover trial with a 10-day wash out period. | No difference in calorie intake (iso-caloric). No difference in physical activity. | ↓ nocturnal glucose AUC TRE group ↓ peak insulin concentrations at breakfast in TRE group ↓ peak glucose concentration at breakfast in TRE group | 100% adherence. Improvement of subjective feelings (well-being and satisfaction) ↓ evening hunger in TRE group |
| R22 Parr et al. 2020 [48] | Low | n = 19 Obese adults with T2D. 50 ± 9 years. | TRE 9/15 every day. 4-week singe-arm non-randomized trial | No difference in calorie intake. Adherence to TRE reduces calorie intake. | NS. | Mean compliance of 72 ± 24% (≅5 days/week). |
| R23 Miguet et al. 2020 [49] | High | n = 105 Overweight and obese adults. 46.5 ± 10.5 years. | dTRE 8/16 every day. 12-week controlled randomized trial. TRE 8/16 vs. control group. | No difference in calorie intake. No measure of physical activity. | ↓ body weight in TRE group (1.17%) compared to baseline that was not significantly different from control group (0.75%). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123770
Adafer R, Messaadi W, Meddahi M, Patey A, Haderbache A, Bayen S, Messaadi N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients. 2020; 12(12):3770. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123770
Chicago/Turabian StyleAdafer, Réda, Wassil Messaadi, Mériem Meddahi, Alexia Patey, Abdelmalik Haderbache, Sabine Bayen, and Nassir Messaadi. 2020. "Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health" Nutrients 12, no. 12: 3770. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123770