Plasma Glutamine Levels in Relation to Intensive Care Unit Patient Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Measurements
2.3. Anthropometric Measurements
2.4. Clinical and Medical Parameters
2.5. Dietary Intake
2.6. Plasma Glutamine
2.7. Data Analysis
2.8. Ethical Principles
3. Results
3.1. Medical Data
3.2. Anthropometry
3.3. Dietary Intake
3.4. Biochemical Data
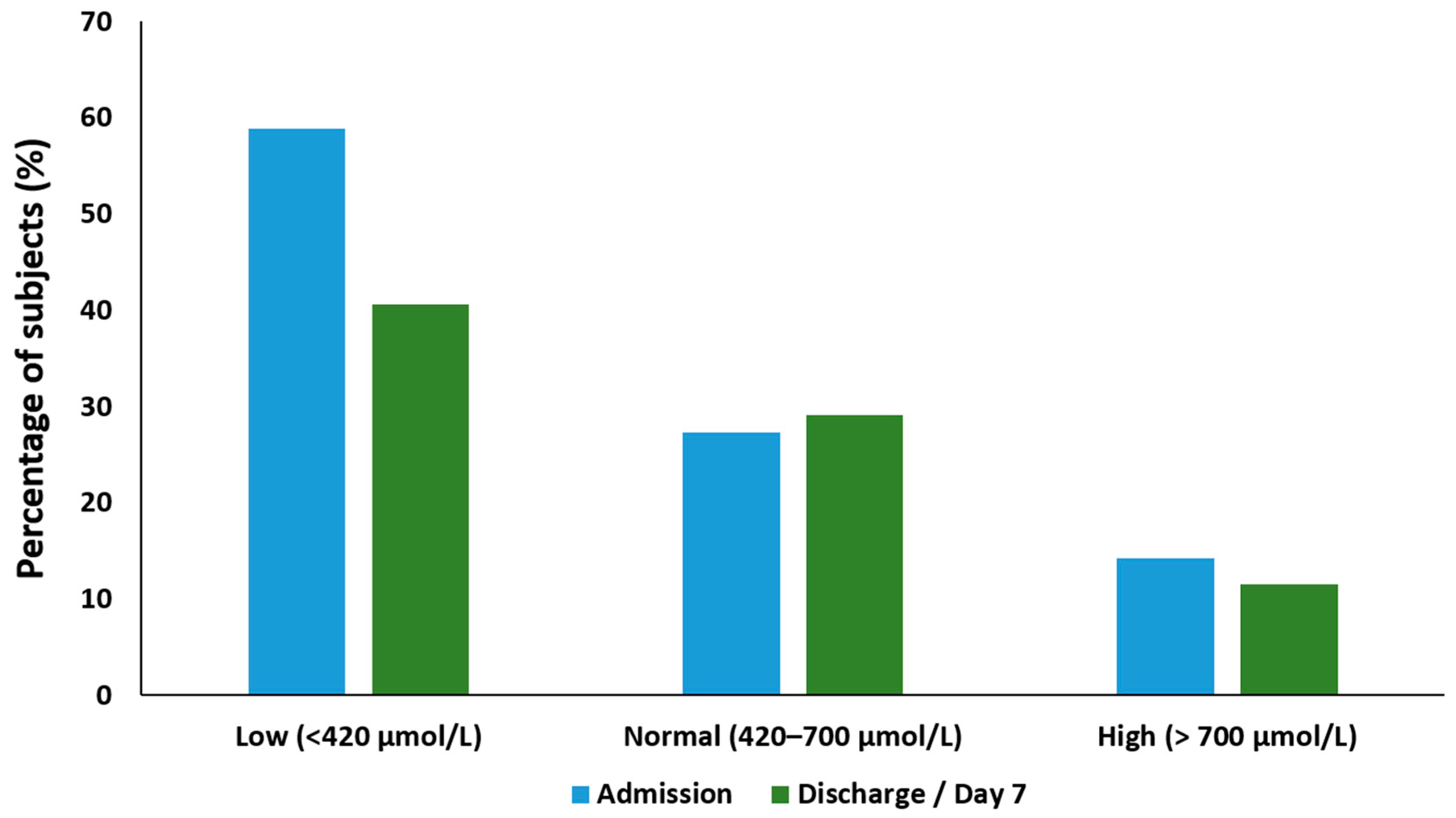
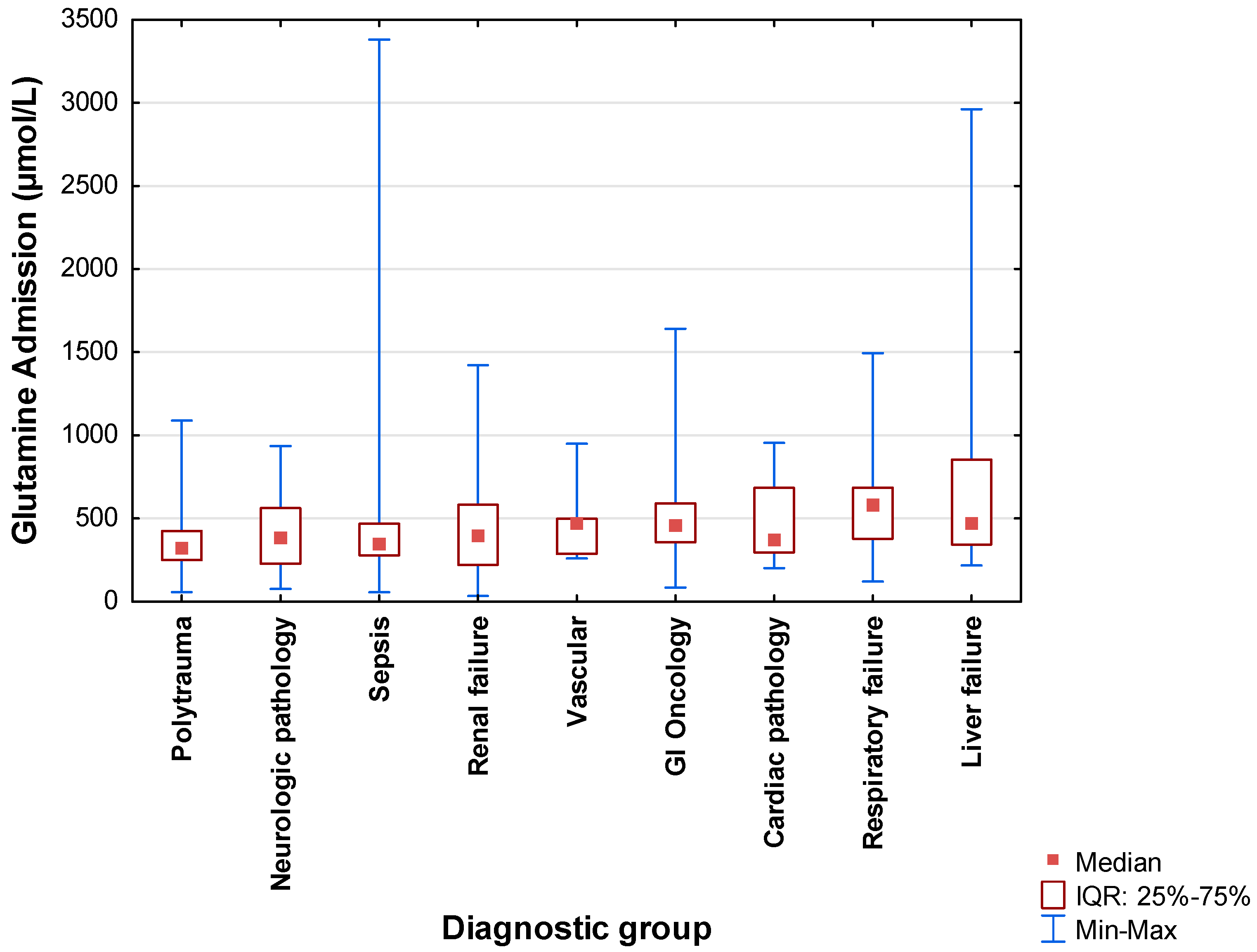
3.5. Plasma Glutamine
3.6. Changes in Glutamine Status from Admission to Discharge
3.7. Associations between Plasma Glutamine and Various Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCarthy, M.S.; Martindale, R.G. Immunonutrition in Critical Illness: What Is the Role? Nutr. Clin. Pract. 2018, 33, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Novak, F.; Heyland, D.K.; Avenell, A.; Drover, J.W.; Su, X. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit. Care Med. 2002, 30, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Chung, X.J.; Yang, C.Y.; Lau, H.L. A meta-analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Burns 2013, 39, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Bollhalder, L.; Pfeil, A.M.; Tomonaga, Y.; Schwenkglenks, M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin. Nutr. 2013, 32, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Tian, W.; Wang, W.; Huang, Q.; Zhao, R.; Zhao, Y.; Li, Q.; Li, J. The Impact of Perioperative Glutamine- supplemented Parenteral Nutrition on Outcomes of Patients Undergoing Abdominal Surgery: A Meta-analysis of Randomized Clinical Trials. Am. Surg. 2013, 79, 506–513. [Google Scholar]
- Weitzel, L.R.B.; Wischmeyer, P.E. Glutamine in critical illness: The time has come, the time is now. Crit. Care Clin. 2010, 26, 515–525. [Google Scholar] [CrossRef]
- Stehle, P.; Ellger, B.; Kojic, D.; Feuersenger, A.; Schneid, C.; Stover, J.; Scheiner, D.; Westphal, M. Glutamine dipeptide-supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: A systematic evaluation of randomised controlled trials. Clin. Nutr. ESPEN 2017, 17, 75–85. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; He, H.; Xie, J.; Cai, S.-X.; Liu, A.; Wang, H.; Qiu, H.-B. The effect of glutamine therapy on outcomes in critically ill patients: A meta-analysis of randomized controlled trials. Crit. Care 2014, 18, R8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, Z.; Nolan, M.T.; Jiang, H.; Han, H.; Yu, K.; Li, H.; Jie, B.; Liang, X. The impact of Glutamine Dipeptide-Supplemented Parenteral Nutrition on Outcomes of Surgical Patients: A meta-analysis of randomized clinical trials. JPEN J. Parenter. Enter. Nutr. 2010, 34, 521–529. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Dhaliwal, R.; Mccall, M.; Ziegler, T.R.; Heyland, D.K. Parenteral glutamine supplementation in critical illness: A systematic review. Crit. Care 2014, 18, R76. [Google Scholar] [CrossRef] [Green Version]
- Pradelli, L.; Povero, M.; Muscaritoli, M.; Eandi, M. Updated cost-effectiveness analysis of supplemental glutamine for parenteral nutrition of intensive-care patients. Eur. J. Clin. Nutr. 2014, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyland, D.; Muscdere, J.; Wischmeyer, P.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Ph, D.; Day, A.G.; et al. A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. NEJM 2013, 368, 1489–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zanten, A.R.H.; Sztark, F.; Kaisers, U.X.; Zielmann, S.; Felbinger, T.W.; Sablotzki, A.R.; De Waele, J.J.; Timsit, J.-F.; Honing, M.L.H.; Keh, D.; et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 312, 514–524. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R. Role of glutamine supplementation in critical illness given the results of the REDOXS study. JPEN J. Parenter. Enter. Nutr. 2013, 37, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, Å.; Wernerman, J. Glutamine and glutathione at ICU admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar]
- Tsujimoto, T.; Shimizu, K.; Hata, N.; Takagi, T. Both high and low plasma glutamine levels predict mortality in critically ill patients. Surg. Today 2017, 47, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Treskes, M.; Van der Spoel, H.J.I.; Zandstra, D.F. Plasma glutamine depletion and patient outcome in acute ICU admissions. Int. Care Med. 2001, 27, 84–90. [Google Scholar] [CrossRef]
- Ling, H.H.; Pan, Y.; Fan, C.; Tseng, W.; Huang, J. Clinical Significance of Serum Glutamine Level in Patients with Colorectal Cancer. Nutrients 2019, 11, 898. [Google Scholar] [CrossRef] [Green Version]
- Hofman, Z.; Swinkels, S.; Zanten, A.R.H. Van Glutamine, fish oil and antioxidants in critical illness: MetaPlus trial post hoc safety analysis. Ann. Intensive Care 2016, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Buter, H.; Koopmans, M.; Kemperman, R.; Jekel, L.; Boerma, C. Plasma glutamine levels before cardiac surgery are related to post-surgery infections; an observational study. J. Cardiothorac. Surg. 2016, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Nienaber, A.; Dolman, R.C.; Van Graan, A.E.; Blaauw, R. Prevalence of glutamine deficiency in ICU patients: A cross-sectional analytical study. Nutr. J. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buter, H.; Bakker, A.J.; Kingma, W.P.; Koopmans, M.; Boerma, E.C. Plasma glutamine levels in patients after non-elective or elective ICU admission: An observational study. BMC Anesthesiol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helling, G.; Wahlin, S.; Smedberg, M.; Pettersson, L.; Tjäder, I. Plasma Glutamine Concentrations in Liver Failure. PLoS ONE 2016, 11, e0150440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyland, D.K.; Elke, G.; Cook, D.; Berger, M.M.; Wischmeyer, P.E.; Albert, M.; Muscedere, J.; Jones, G.; Day, A.G. Glutamine and Antioxidants in the Critically Ill Patient: A Post Hoc Analysis of a Large-Scale Randomized Trial. JP 2015, 39, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Perez-Barcena, J.; Marse, P.; Zabalegui-Perez, A.; Corral, E.; Herran-Monge, R.; Gero-Escapa, M.; Cervera, M.; Llompart-Pou, J.A.; Ayestaran, I.; Raurich, J.M.; et al. A randomized trial of intravenous glutamine supplementation in trauma ICU patients. Int. Care Med. 2014, 40, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, L.; Rydén, S.; Smedberg, M.; Tjader, I.; Rooyackers, O.; Wernerman, J. Validation of a point-of-care instrument for bedside glutamine screening in the intensive care unit. Clin. Nutr. 2017, 36, 186–190. [Google Scholar] [CrossRef]
- Cynober, L.; De Bandt, J.-P. Glutamine in the intensive care unit. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 98–104. [Google Scholar] [CrossRef]
- Wernerman, J. Glutamine supplementation. Ann. Intensive Care 2011, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Wernerman, J. Glutamine supplementation to critically ill patients? Crit. Care 2014, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Rooyackers, O.; Smedberg, M.; Tjäder, I.; Norberg, A.; Wernerman, J. Endogenous glutamine production in critically ill patients: The effect of exogenous glutamine supplementation. Crit. Care 2014, 18, R72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, P.; Reintam, A.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Carlos, J.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClave, S.A.; Kushner, R.; Van Way, C.W.; Cave, M.; DeLegge, M.; Dibaise, J.; Dickerson, R.; Drover, J.; Frazier, T.H.; Fujioka, K.; et al. Nutrition Therapy of the Severely Obese, Critically Ill Patient: Summation of Conclusions and Recommendations. JPEN J. Parenter Enter. Nutr. 2011, 35, 88S–96S. [Google Scholar] [CrossRef] [PubMed]
- Elke, G.; Hartl, W.H.; Kreymann, K.G.; Adolph, M.; Felbinger, T.W.; Graf, T.; de Heer, G.; Heller, A.R.; Kampa, U.; Mayer, K.; et al. Clinical Nutrition in Critical Care Medicine–Guideline of the German Society for Nutritional Medicine (DGEM). Clin. Nutr. ESPEN 2019, 33, 220–275. [Google Scholar] [CrossRef] [PubMed]

| Variables | Unit | |
|---|---|---|
| n | Percentage (%) | |
| Gender (n = 330) | ||
| Male | 186 | 56.4 |
| Female | 144 | 43.6 |
| Disease category (n = 330) | ||
| Medical | 147 | 47.6 |
| Surgical | 173 | 52.4 |
| Primary diagnosis (n = 330) | ||
| Sepsis | 118 | 35.8 |
| Polytrauma | 62 | 18.8 |
| Gastrointestinal oncology | 42 | 12.7 |
| Liver failure | 28 | 8.5 |
| Renal failure | 24 | 7.3 |
| Neurologic pathology | 20 | 6.1 |
| Respiratory failure | 20 | 6.1 |
| Cardiac pathology | 9 | 2.7 |
| Vascular | 7 | 2.1 |
| Mechanical ventilation on admission (n = 330) | ||
| Yes | 223 | 67.6 |
| No | 107 | 32.4 |
| HIV positive (n = 250) | ||
| Yes | 45 | 18.0 |
| No | 205 | 82.0 |
| Tuberculosis positive (n = 181) | ||
| Yes | 9 | 5.0 |
| No | 172 | 95.0 |
| BMI, categories (kg/m2) (n = 328) | ||
| Underweight (<18.5 kg/m2) | 14 | 4.3 |
| Normal weight (18.5–24.9 kg/m2) | 126 | 38.4 |
| Overweight (25.0–29.9 kg/m2) | 106 | 32.3 |
| Obese (30.0–39.9 kg/m2) | 82 | 25 |
| Mean ± SD | Median (IQR) | |
| Age (years) | 47.4 ± 16.6 | 46.8 (32.0–60.2) |
| Illness severity indicators | ||
| APACHE II score (Admission) (n = 330) | 18.6 ± 8.6 | 18.0 (11.0–25.0) |
| SOFA score (Admission) (n = 330) | 7.1 ± 3.8 | 7.0 (4.0–10.0) |
| Length of stay (days) (n = 330) | ||
| ICU | 9.5 ± 8.7 | 7.0 (4.0–11.0) |
| Hospital | 18.6 ± 14.9 | 14.0 (8.0–26.0) |
| Biochemical Variable | Normal Range | Unit | Admission | Day 7/Discharge | ||
|---|---|---|---|---|---|---|
| n | n | |||||
| Albumin (g/L) | 35–52 | Mean ± SD Median IQR | 25.8 ± 7.7 26.0 21.0–31.0 | 318 | 25.0 ± 13.9 24.0 19.0–29.0 | 280 |
| Urea (mmol/L) | 2.6–7.0 | Mean ± SD Median IQR | 11.9 ± 16.6 6.9 4.5–14.6 | 324 | 12.7 ± 12.7 7.8 4.5–14.1 | 289 |
| Creatinine (µmol/L) | 60–100 | Mean ± SD Median IQR | 206.7 ± 290.4 103.0 68.0–197.5 | 324 | 167.5 ± 218.9 72.0 55.0–147.0 | 289 |
| Total bilirubin (µmol/L) | 0–21 | Mean ± SD Median IQR | 25.1 ± 47.1 11.0 7.0–3.0 | 322 | 23.2 ± 44.3 10.0 6.0–20.0 | 281 |
| AST (u/L) | 8–20 | Mean ± SD Median IQR | 289.9 ± 1105.7 53.0 28.0–153.0 | 321 | 84.3 ± 127.1 47.5 29.0–83.0 | 278 |
| ALT (u/L) | 5–40 | Mean ± SD Median IQR | 195.4 ± 675.3 34.0 19.0–87.0 | 322 | 98.6 ± 155.7 49.0 20.0–104.0 | 279 |
| LDH (U/L) | 140–280 | Mean ± SD Median IQR | 671.5 ± 1167.1 385.0 269.0–604.0 | 303 | 453.9 ± 332.7 379.0 256.0–517.0 | 265 |
| CRP (mg/L) | <10 | Mean ± SD Median IQR | 150.1 ± 133.9 117.0 35.5–251.0 | 322 | 122.6 ± 139.2 90.0 46.0–173.2 | 287 |
| Parameter | Glutamine <420 µmol/L (n = 193) | Glutamine ≥420 µmol/L (n = 137) | p-Value |
|---|---|---|---|
| Disease category (n (%)) | |||
| Medical | 90 (57.3) | 67 (42.7) | 0.683; Chi2 = 0.16 * |
| Surgical | 103 (59.5) | 70 (40.5) | |
| RVD status (n = 250) (n (%)) | |||
| Positive | 31 (68.9) | 14 (31.1) | 0.06; Chi2 = 3.36 * |
| Negative | 111 (54.2) | 94 (45.8) | |
| Tuberculosis status (n = 181) (n (%)) | |||
| Positive | 8 (88.9) | 1 (11.1) | 0.029; Chi2 = 4.75 * |
| Negative | 94 (54.7) | 78 (45.3) | |
| Mechanical ventilation on admission (n = 324) (n (%)) | |||
| Yes | 145 (65.6) | 76 (34.4) | <0.001; Chi2 = 12.65 * |
| No | 46 (44.7) | 57 (55.3) | |
| Severity of disease (mean ± SD; median; IQR) | |||
| APACHE II score (n = 327) | 19.8 ± 8.9; 20.0; 12.0–26.0 | 16.9 ± 7.9; 16.0; 10.0–22.0 | 0.003 *** |
| SOFA score Admission (n = 326) | 7.6 ± 3.8; 7.0; 5.0–11.0 | 6.3 ± 3.6; 6.0; 3.0–8.0 | 0.003 *** |
| SOFA score Day 7 (n = 291) | 4.8 ± 4.2; 4.0; 1.5–7.0 | 4.5 ± 4.3; 3.0; 1.0–6.0 | 0.573 *** |
| Length of stay (days) (mean ± SD; median; IQR) | |||
| ICU | 9.5 ± 6.9; 7.0; 4.0–11.0 | 9.5 ± 10.7; 6.0; 4.0–11.0 | 0.966 ** |
| Hospital | 19.7 ± 15.5; 15.0; 8.0–28.0 | 17.1 ± 14.1; 13.0; 8.0–23.0 | 0.125 ** |
| Number of complications (n = 305) (mean ± SD; median; IQR) | 2.8 ± 3.1; 2.0; 1.0–4.0 | 2.5 ± 3.1; 2.0; 1.0–3.0 | 0.460 ** |
| Mortality during hospitalization (n = 316) (n (%)) | |||
| Yes | 50 (64.1) | 28 (35.9) | 0.305; Chi2 = 1.05 * |
| No | 137 (57.6) | 101 (42.4) | |
| Nutrition support (n (%)) | |||
| Yes | 156 (62.2) | 95 (37.8) | 0.016; Chi2 = 5.74 * |
| No | 37 (46.8) | 42 (53.2) | |
| Parameter | Unit | Baseline Data | Discharge/Day 7 data | ||||
|---|---|---|---|---|---|---|---|
| Glutamine <420 µmol/L (n = 193) | Glutamine ≥420 µmol/L (n = 137) | Total (n) p-Value | Glutamine <420 µmol/L (n = 193) | Glutamine ≥420 µmol/L (n = 137) | Total (n) p-Value | ||
| Glutamine (µmol/l) | Mean ± SD | 280.5 ± 93.9 | 724.6 ± 397.3 | n = 330 <0.001 * | 419.1 ± 230.9 | 630.5 ± 772.1 | n = 268 <0.001 * |
| Median | 299.50 | 617.20 | 383.1 | 484.7 | |||
| IQR | 218.60–352.70 | 497.15–815.96 | 293.3–482.8 | 383.3–642.5 | |||
| Albumin (g/L) | Mean ± SD | 24.3 ± 7.5 | 28.0 ± 7.5 | n = 318 <0.001 * | 24.1 ± 17.5 | 26.2 ± 6.8 | n = 280 0.208 * |
| Median | 24.0 | 29.0 | 23.0 | 26.0 | |||
| IQR | 20.0–30.0 | 22.0–33.0 | 18.0–27.0 | 22.0–31.0 | |||
| Urea (mmol/L) | Mean ± SD | 13.9 ± 20.4 | 8.9 ± 7.9 | n = 324 0.008 * | 14.1 ± 12.9 | 10.8 ± 12.1 | n = 289 0.028 * |
| Median | 8.0 | 6.4 | 8.4 | 6.9 | |||
| IQR | 5.0–16.3 | 3.6–11.2 | 4.4–21.5 | 4.5–11.1 | |||
| Creatinine (µmol/L) | Mean ± SD | 237.4 ± 341.6 | 162.8 ± 187.4 | n = 324 0.023 * | 197.9 ± 250.9 | 125.9 ± 157.2 | n = 289 0.006 * |
| Median | 111.0 | 93.0 | 74.0 | 69.5 | |||
| IQR | 70.0–250.0 | 62.0–141.0 | 56.0–229.0 | 54.0–107.0 | |||
| Total bilirubin (µmol/L) | Mean ± SD | 20.9 ± 41.5 | 31.1 ± 53.7 | n = 322 0.054 * | 18.9 ± 38.8 | 29.0 ± 50.8 | n = 281 0.059 * |
| Median | 11.0 | 12.0 | 10.0 | 10.0 | |||
| IQR | 7.0–20.0 | 6.0–27.0 | 6.0–18.0 | 6.0–22.0 | |||
| AST (u/L) | Mean ± SD | 271.4 ± 1186.2 | 316.3 ± 981.1 | n = 321 0.721 * | 94.2 ± 154.8 | 70.9 ± 73.3 | n = 278 0.132 * |
| Median | 61.0 | 44.0 | 46.0 | 52.0 | |||
| IQR | 31.0–158.0 | 25.0–129.5 | 28.5–85.0 | 31.0–78.0 | |||
| ALT (u/L) | Mean ± SD | 144.8 ± 455.6 | 267.4 ± 896.9 | n=322 0.108 * | 90.8 ± 130.9 | 109.2 ± 184.3 | n = 279 0.332 * |
| Median | 34.0 | 34.0 | 43.0 | 56.0 | |||
| IQR | 19.0–82.0 | 19.0–93.0 | 19.0–96.0 | 24.0–111.0 | |||
| LDH (u/L) | Mean ± SD | 728.8 ± 1208.1 | 586.5 ± 1102.9 | n = 303 0.298 * | 493.1 ± 361.2 | 402.9 ± 284.9 | n = 265 0.029 * |
| Median | 432.0 | 327.5 | 398.5 | 316.0 | |||
| IQR | 293.0–648.0 | 244.0–546.0 | 290.0–573.0 | 230.0–491.0 | |||
| CRP (mg/L) | Mean ± SD | 190.1 ± 135.9 | 93.3 ± 108.2 | n = 322 <0.001 * | 144.3 ± 164.5 | 93.2 ± 87.1 | n = 287 0.002 * |
| Median | 172.0 | 53.0 | 114.5 | 72.6 | |||
| IQR | 67.0–300.0 | 13.0–125.0 | 56.0–187.0 | 29.9–135.9 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaauw, R.; Nel, D.G.; Schleicher, G.K. Plasma Glutamine Levels in Relation to Intensive Care Unit Patient Outcome. Nutrients 2020, 12, 402. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020402
Blaauw R, Nel DG, Schleicher GK. Plasma Glutamine Levels in Relation to Intensive Care Unit Patient Outcome. Nutrients. 2020; 12(2):402. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020402
Chicago/Turabian StyleBlaauw, Renée, Daan G. Nel, and Gunter K. Schleicher. 2020. "Plasma Glutamine Levels in Relation to Intensive Care Unit Patient Outcome" Nutrients 12, no. 2: 402. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020402





