Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells
Abstract
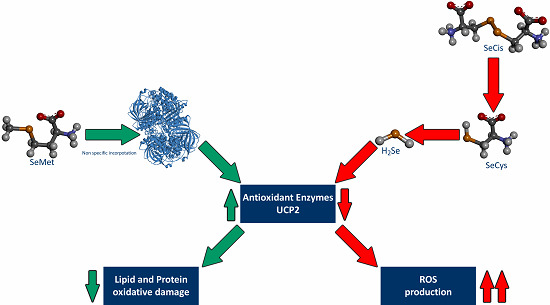
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Cell Culture and Treatments
2.3. Cell Viability Assay
2.4. Measurement of ROS Production
2.5. Western Blot
2.6. Measurement of Carbonyl Groups
2.7. Measurement of 4-Hydroxy-2Nonenal Adducts
2.8. Statistical Analysis
3. Results
3.1. Effects of Increasing Concentrations of Selenoamino Acids on Cell Viability and H2O2 Production
3.2. Effects of Selenoamino Acids on Antioxidant Enzymes and UCP2 Protein Levels
3.3. Effects of Selenoamino Acids Treatment on Protein and Lipid Oxidative Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Jackson, M.I.; Combs, G.F. Selenium and anticarcinogenesis: Underlying mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sosnoski, D.M.; Gandhi, U.H.; Novinger, L.J.; Prabhu, K.S.; Mastro, A.M. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis 2009, 30, 1941–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrauzer, G.N. Anticarcinogenic effects of selenium. Cell. Mol. Life Sci. 2000, 57, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 2005, 1055, 93–135. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Valle, A.; Company, M.M.; Garau, I.; Oliver, J.; Roca, P. Estrogen down-regulates uncoupling proteins and increases oxidative stress in breast cancer. Free Radic. Biol. Med. 2010, 48, 506–512. [Google Scholar] [CrossRef]
- Miró, A.M.; Sastre-Serra, J.; Pons, D.G.; Valle, A.; Roca, P.; Oliver, J. 17β-Estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2011, 123, 133–139. [Google Scholar] [CrossRef]
- Nadal-Serrano, M.; Sastre-Serra, J.; Pons, D.G.; Miró, A.M.; Oliver, J.; Roca, P. The ERalpha/ERbeta ratio determines oxidative stress in breast cancer cell lines in response to 17Beta-estradiol. J. Cell. Biochem. 2012, 113, 3178–3182. [Google Scholar] [CrossRef]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Valle, A.; Oliver, J.; Roca, P. UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free Radic. Biol. Med. 2015, 86, 67–77. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Ong, T.P. Selenium and Breast Cancer Risk: Focus on Cellular and Molecular Mechanisms. Adv. Cancer Res. 2017, 136, 173–192. [Google Scholar]
- Ross, A.C.; Cousins, R.J.; Caballero, B.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Wolters Kluwer Health, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; ISBN 978-1-60-547461-8. [Google Scholar]
- Chun, O.K.; Floegel, A.; Chung, S.J.; Chung, C.E.; Song, W.O.; Koo, S.I. Estimation of Antioxidant Intakes from Diet and Supplements in U.S. Adults. J. Nutr. 2010, 140, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Coates, P.; Betz, J.M.; Blackman, M.R.; Cragg, G.; Levine, M.; Moss, J.; White, J. Encyclopedia of Dietary Supplements Encyclopedia of Dietary Supplements, 2nd ed.; Taylor & Francis: Abingdon, UK, 2010; ISBN 9781439819289. [Google Scholar]
- Erdman, J.W.; MacDonald, I.; Zeisel, S.H. Present Knowledge in Nutrition, 10th ed.; International Life Sciences Institute: Washington, DC, USA, 2012; ISBN 9780470959176. [Google Scholar]
- Medicine, I. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 978-0-309-06935-9. [Google Scholar]
- Davis, C.D. Selenium Supplementation and Cancer Prevention. Curr. Nutr. Rep. 2012, 1, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Ip, C. Lessons from Basic Research in Selenium and Cancer Prevention. J. Nutr. 1998, 128, 1845–1854. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R. Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 181–197. [Google Scholar] [CrossRef]
- Richards, W.L.; Song, M.K.; Krutzsch, H.; Evarts, R.P.; Marsden, E.; Thorgeirsson, S.S. Measurement of cell proliferation in microculture using Hoechst 33342 for the rapid semiautomated microfluorimetric determination of chromatin DNA. Exp. Cell Res. 1985, 159, 235–246. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Ahmiane, Y.; Roca, P.; Oliver, J.; Pons, D.G. Xanthohumol, a hop-derived prenylflavonoid present in beer, impairs mitochondrial functionality of SW620 colon cancer cells. Int. J. Food Sci. Nutr. 2019, 70, 396–404. [Google Scholar] [CrossRef]
- Stewart, M.S.; Spallholz, J.E.; Neldner, K.H.; Pence, B.C. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic. Biol. Med. 1999, 26, 42–48. [Google Scholar] [CrossRef]
- Yan, L.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993, 45, 429–437. [Google Scholar]
- Liu, J.; Wang, S.; Zhang, Q.; Li, X.; Xu, S. Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics 2020, 12, 54–64. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Dickson, R.C.; Tappel, A.L. Reduction of Selenocystine by Cysteine or Gluthathione. Arch. Biochem. Biophys. 1969, 130, 547–550. [Google Scholar] [CrossRef]
- Li, Z.; Carrier, L.; Belame, A.; Thiyagarajah, A.; Salvo, V.A.; Burow, M.E.; Rowan, B.G. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res. Treat. 2009, 118, 33–43. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wong, Y.S. Selenocystine induces reactive oxygen species-mediated apoptosis in human cancer cells. Biomed. Pharmacother. 2009, 63, 105–113. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S. Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. J. Agric. Food Chem. 2008, 56, 10574–10581. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S. Selenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int. J. Biochem. Cell Biol. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Jackson, M.I.; Combs, G.F. Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Berry, M.J., Gladyshev, V.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9781461410256. [Google Scholar]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Shilo, S.; Aharoni-Simon, M.; Tirosh, O. Selenium Attenuates Expression of MnSOD and Uncoupling Protein 2 in J774.2 Macrophages: Molecular Mechanism for Its Cell-Death and Antiinflammatory Activity. Antioxid. Redox Signal. 2005, 7, 276–286. [Google Scholar] [CrossRef]
- Tanaka, H.; Esaki, N.; Soda, K. A versatile bacterial enzyme: L-methionine γ-lyase. Enzyme Microb. Technol. 1985, 7, 530–537. [Google Scholar] [CrossRef]
- Miki, K.; Xu, M.; Gupta, A.; Ba, Y.; Tan, Y.; Al-Refaie, W.; Bouvet, M.; Makuuchi, M.; Moossa, A.R.; Hoffman, R.M. Methioninase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res. 2001, 61, 6805–6810. [Google Scholar]
- Okuno, T.; Ueno, H.; Nakamuro, K. Cystathionine γ-Lyase Contributes to Selenomethionine Detoxification and Cytosolic Glutathione Peroxidase Biosynthesis in Mouse Liver. Biol. Trace Elem. Res. 2006, 109, 155–171. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030865
Pons DG, Moran C, Alorda-Clara M, Oliver J, Roca P, Sastre-Serra J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients. 2020; 12(3):865. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030865
Chicago/Turabian StylePons, Daniel Gabriel, Carmen Moran, Marina Alorda-Clara, Jordi Oliver, Pilar Roca, and Jorge Sastre-Serra. 2020. "Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells" Nutrients 12, no. 3: 865. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030865