Kokumi Taste Active Peptides Modulate Salt and Umami Taste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of FIIm and FIIim from JGN
2.2. CT Taste Nerve Recordings
2.3. Behavior Studies in Mice
2.4. Human Sensory Evaluation
2.4.1. Salt Sensory Evaluation
2.4.2. Umami Sensory Evaluation
3. Results and Discussion
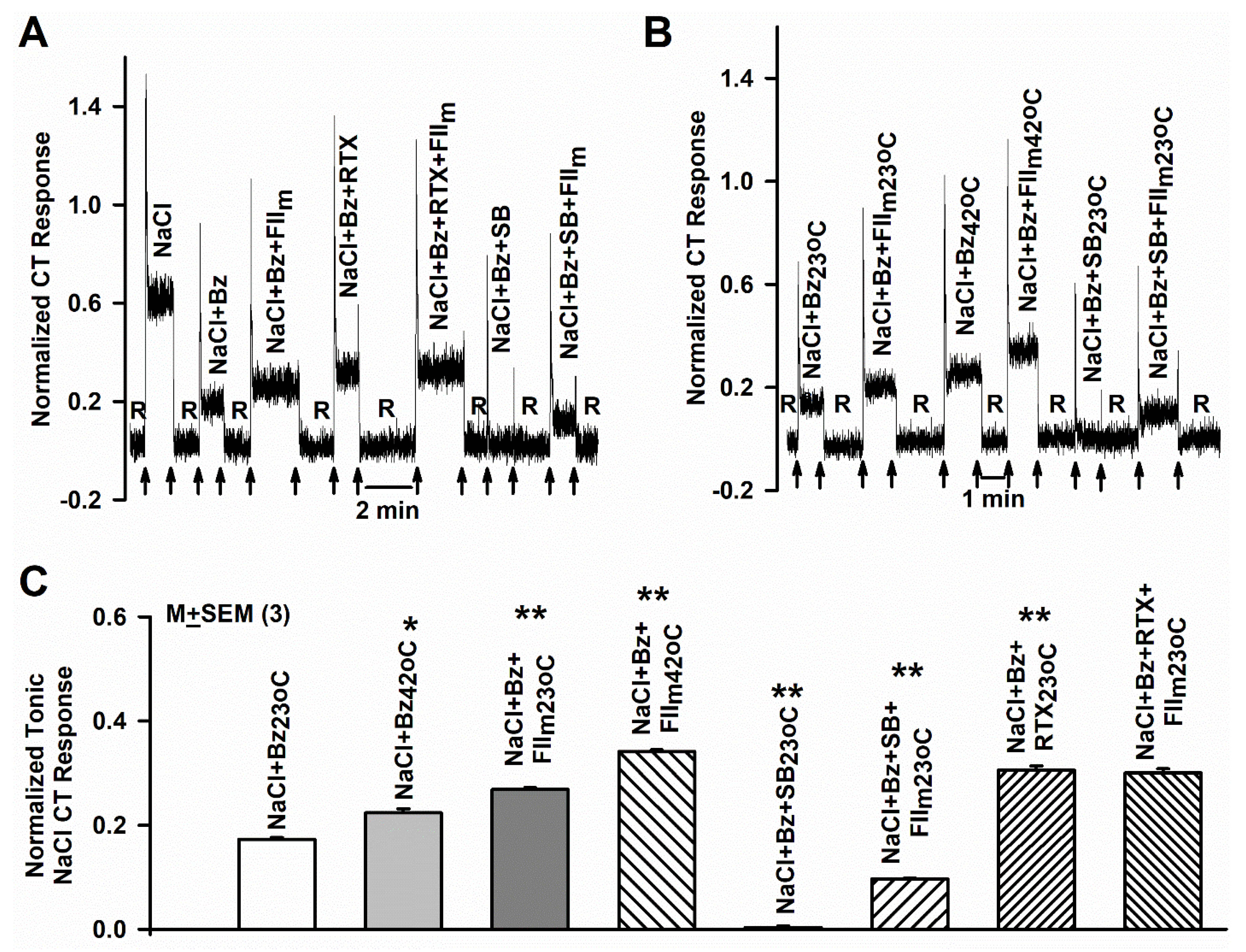
3.1. Effect of FIIm and FIIim on the Bz-insensitive NaCl CT Response
3.2. Effect of SB and FIIm on the Bz-insensitive NaCl CT Response
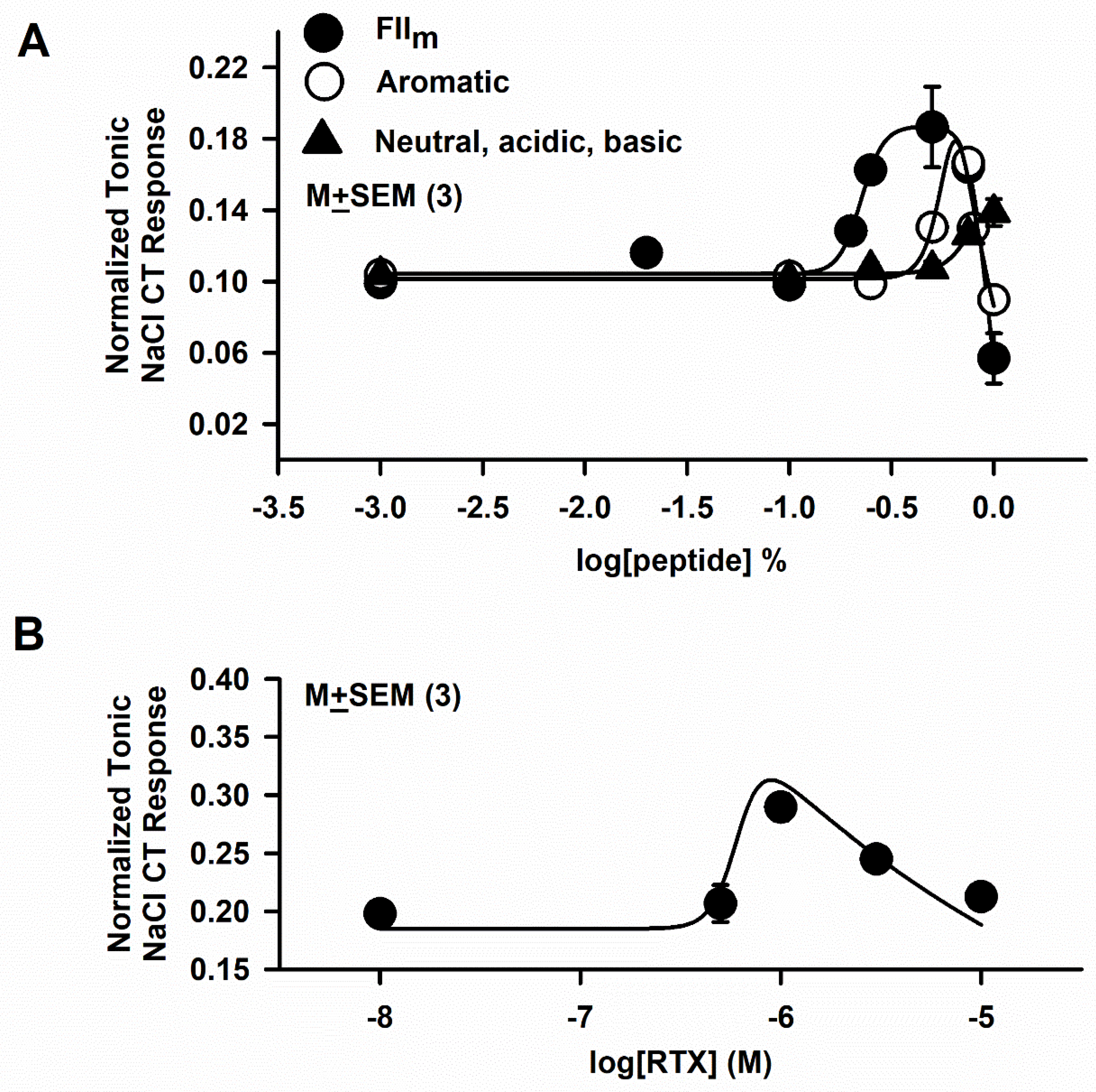
3.3. Effect of RTX and FIIm on the NaCl + Bz CT Response
3.4. Effect of Elevated Temperature and FIIm on the NaCl + Bz CT Response
3.5. Effect of FIIm sub-fractions of Different Molecular Weights (FIIm(a-d)) on the NaCl + Bz CT Response
3.6. Effect of FIIm sub-fractions (Neutral, Acidic, Basic and Aromatic) on the NaCl + Bz CT Response
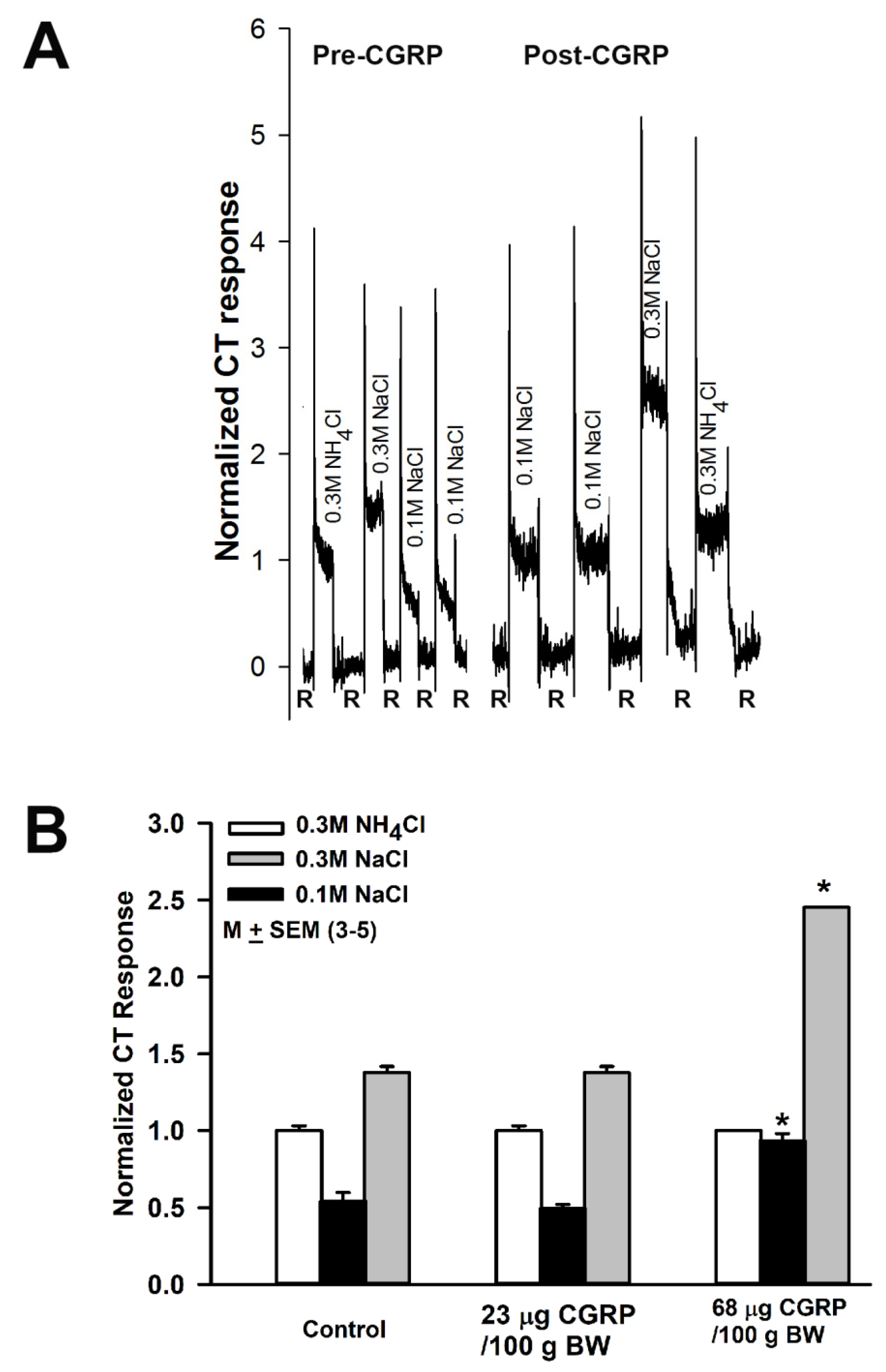
3.7. Effect of Calcitonin Gene Related Peptide (CGRP) on NaCl CT Responses
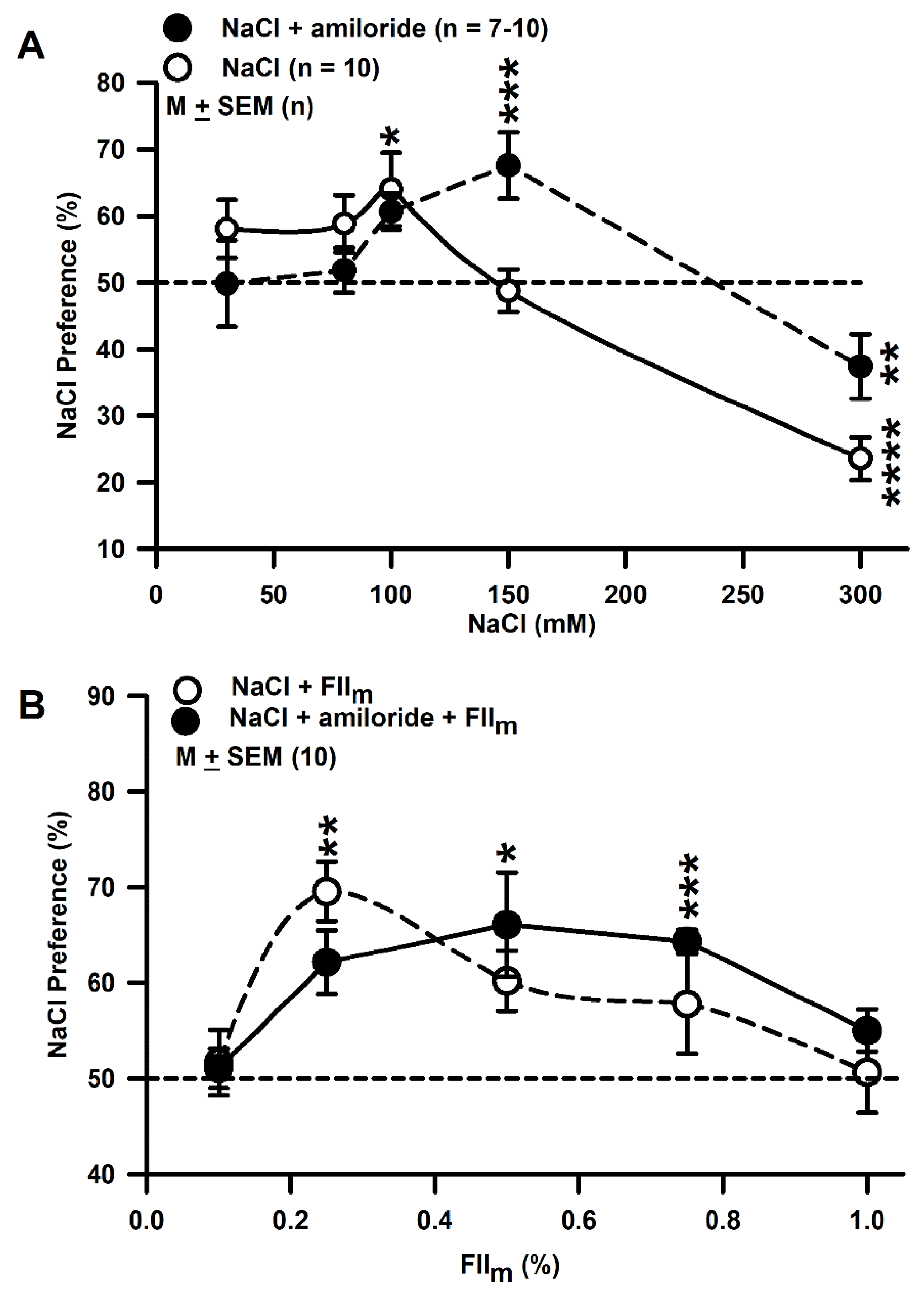
3.8. Behavioral Studies with Mice
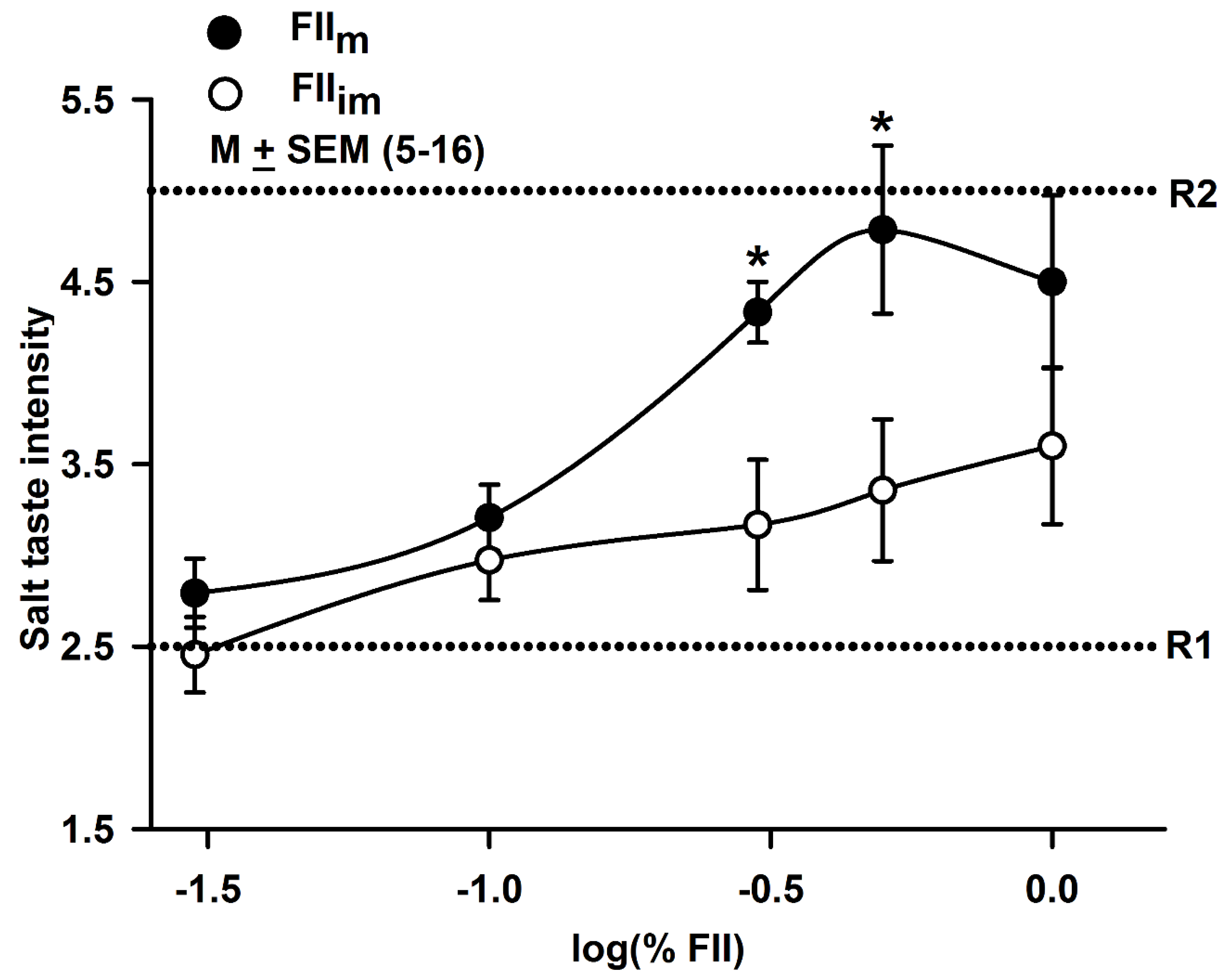
3.9. Effect of FIIm on Salt Taste in Human
3.10. Effect of FIIm on the Rat CT Response to Glutamate
3.11. Effect of FIIm on Umami Taste in Human
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, B.C.; Sukumaran, S.K.; Margolskee, R.F.; Bachmanov, A.A. Amiloride-Insensitive Salt Taste Is Mediated by Two Populations of Type III Taste Cells with Distinct Transduction Mechanisms. J. Neurosci. 2016, 36, 1942–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roebber, J.K.; Roper, S.D.; Chaudhari, N. The Role of the Anion in Salt (NaCl) Detection by Mouse Taste Buds. J. Neurosci. 2019, 39, 6224–6232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, K.; Nakanishi, M.; Ishidate, F.; Iwata, K.; Taruno, A. All-Electrical Ca2+-Independent Signal Transduction Mediates Attractive Sodium Taste in Taste Buds. Neuron 2020, 106, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour Sensing from the Tongue to the Brain. Cell 2019, 179, 392–402. [Google Scholar] [CrossRef]
- Teng, B.; Wilson, C.E.; Tu, Y.H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Biol. 2019, 29, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Keast, S.J.R.; Breslin, P.A.S. An overview of binary taste-taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef]
- Zhang, F.; Klebansky, B.; Fine, R.M.; Xu, H.; Pronin, A.; Liu, H.; Tachdjian, C.; Li, X. Molecular mechanism for the umami taste synergism. Proc. Natl. Acad. Sci. USA 2008, 105, 20930–20934. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Son, H.J.; Kim, Y.; Misaka, T.; Rhyu, M.R. Umami-bitter interactions: The suppression of bitterness by umami peptides via human bitter taste receptor. Biochem. Biophys. Res. Commun. 2015, 456, 586–590. [Google Scholar] [CrossRef]
- Servant, G.; Tachdjian, C.; Tang, X.Q.; Werner, S.; Zhang, F.; Li, X.; Kamdar, P.; Petrovic, G.; Ditschun, T.; Java, A.; et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 4746–4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuBois, G.E.; Prakash, I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Sakaguchi, M.; Hirayama, K.; Miyajima, R.; Kimizuka, A. Characteristic Flavor Constituents in Water Extract of Garlic. Agric. Biol. Chem. 1990, 54, 163–169. [Google Scholar]
- Maruyama, Y.; Yasuda, R.; Kuroda, M.; Eto, Y. Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS ONE 2012, 7, e34489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Tsubuku, T.; Miyajima, R. Composition of Sulfur-Containing Components in Onion and Their Flavor Characters. Biosci. Biotechnol. Biochem. 1994, 58, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Yonemitsu, M.; Tsubuku, T.; Sakaguchi, M.; Miyajima, R. Flavor characteristics of glutathione in raw and cooked foodstuffs. Biosci. Biotechnol. Biochem. 1997, 61, 1977–1980. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yao, Y.; Kuang, D.; Hampson, D.R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 2006, 281, 8864–8870. [Google Scholar] [CrossRef] [Green Version]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Maillard, L.C. Réaction de Maillard. Action des acides aminés sur les sucres: Formation des mélanoïdines par voie méthodique. Compte-Rendu De L’académie Des. Sci. 1912, 154, 66–68. [Google Scholar]
- Rhyu, M.R.; Kim, E.Y.; Ogasawara, M.; Nakahisaki, H. Maillard peptides are a clue to the Kokumi taste of a typical Korean soy sauce, Ganjang. In Proceedings of the Association for Chemoreception Sciences, Sarasota, FL, USA, 13–17 April 2005. [Google Scholar]
- DeSimone, J.; Lyall, V. Amiloride-Sensitive Ion Channels. In The Senses: A Comprehensive Reference; Basbaum, A., Kaneko, A., Shepherd, G., Westheimer, G., Stuart, F., Gary, B., Eds.; Olfaction & Taste; Academic Press: San Diego, CA, USA, 2008; Volume 4, pp. 281–288. [Google Scholar]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.; Zuker, C.S. The cells and peripheral representation of sodium taste in mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Finger, T.E.; Rossier, B.C.; Kinnamon, S.C. Epithelial Na+ channel subunits in rat taste cells: Localization and regulation by aldosterone. J. Comp. Neurol. 1999, 405, 406–420. [Google Scholar] [CrossRef]
- Ossebaard, C.A.; Smith, D.V. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: Implications for Na+ receptor mechanisms. Chem. Senses 1995, 20, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.M.; Mogyorosi, A.; Heck, G.L.; DeSimone, J.A.; Santos, C.R.; Clary, R.A.; Lyall, V. Salt-evoked lingual surface potential in humans. J. Neurophysiol. 2003, 90, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, T.; Nakakuki, H.; Tokunaga, C.; Fujii, N.; Egi, M.; Phan, T.H.; Mummalaneni, S.; DeSimone, J.A.; Lyall, V. Effect of Maillard reacted peptides on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t). Chem. Senses 2008, 33, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogasawara, M.; Katsumata, T.; Egi, M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chem. 2006, 99, 600–604. [Google Scholar] [CrossRef]
- Coleman, J.; Williams, A.; Phan, T.H.; Mummalaneni, S.; Melone, P.; Ren, Z.; Zhou, H.; Mahavadi, S.; Murthy, K.S.; Katsumata, T.; et al. Strain differences in the neural, behavioral, and molecular correlates of sweet and salty taste in naive, ethanol- and sucrose-exposed P and NP rats. J. Neurophysiol. 2011, 106, 2606–2621. [Google Scholar] [CrossRef]
- Fujimaki, M.; Arai, S.; Yamashita, M.; Kato, H.; Noguchi, M. Taste Peptide Fractionation from a Fish Protein Hydrolysate. Agric. Biol. Chem. 1973, 37, 2891–2898. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Song, A.Y.; Kim, H.Y.; Kim, S.S.; Tokunaga, C.; Phan, T.-H.T.; Heck, G.L.; DeSimone, J.A.; Lyall, V. Naturally occurring peptides in mature Korean Soy Sauce modulate TRPV1 Variant Salt Taste Receptor. In Proceedings of the Association for Chemoreception Sciences, St. Petersburg, FL, USA, 13–17 April 2011. [Google Scholar]
- DeSimone, J.A.; Lyall, V.; Heck, G.L.; Phan, T.H.; Alam, R.I.; Feldman, G.M.; Buch, R.M. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH(4)Cl chorda tympani taste responses. J. Neurophysiol. 2001, 86, 2638–2641. [Google Scholar] [CrossRef]
- Lyall, V.; Heck, G.L.; Vinnikova, A.K.; Ghosh, S.; Phan, T.H.; Alam, R.I.; Russell, O.F.; Malik, S.A.; Bigbee, J.W.; DeSimone, J.A. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 2004, 558, 147–159. [Google Scholar] [CrossRef]
- Lyall, V.; Heck, G.L.; Vinnikova, A.K.; Ghosh, S.; Phan, T.H.; Desimone, J.A. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem. Senses 2005, 30 (Suppl. 1), i42–i43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyall, V.; Heck, G.L.; Phan, T.H.; Mummalaneni, S.; Malik, S.A.; Vinnikova, A.K.; Desimone, J.A. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J. Gen. Physiol. 2005, 125, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Lyall, V.; Phan, T.H.; Mummalaneni, S.; Mansouri, M.; Heck, G.L.; Kobal, G.; DeSimone, J.A. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J. Neurophysiol. 2007, 98, 1662–1674. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S. The Synergistic Taste Effect of Monosodium Glutamate and Disodium 5′-Inosinate. J. Food Sci. 2006, 32, 473–478. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Rami, H.K.; Jerman, J.C.; Smart, D.; Gill, C.H.; Soffin, E.M.; Luis Hannan, S.; Lappin, S.C.; Egerton, J.; Smith, G.D.; et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 2004, 46, 133–149. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Tordoff, M.G.; Beauchamp, G.K. Voluntary sodium chloride consumption by mice: Differences among five inbred strains. Behav. Genet. 1998, 28, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Descriptive analysis Techniques. In Sensory Evaluation Techniques, 2nd ed.; Meilgaard, M., Civille, G.V., Carr, B.T., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 119–142. [Google Scholar]
- Lyall, V.; Phan, T.H.; Mummalaneni, S.; Melone, P.; Mahavadi, S.; Murthy, K.S.; DeSimone, J.A. Regulation of the benzamil-insensitive salt taste receptor by intracellular Ca2+, protein kinase C, and calcineurin. J. Neurophysiol. 2009, 102, 1591–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyall, V.; Phan, T.H.; Ren, Z.; Mummalaneni, S.; Melone, P.; Mahavadi, S.; Murthy, K.S.; DeSimone, J.A. Regulation of the putative TRPV1t salt taste receptor by phosphatidylinositol 4,5-bisphosphate. J. Neurophysiol. 2010, 103, 1337–1349. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.F.; Lee, J.H.; Yoo, S.B.; Moon, Y.W.; Jahng, J.W. Intra-oral pre-treatment with capsaicin increases consumption of sweet solutions in rats. Nutr. Neurosci. 2009, 12, 149–154. [Google Scholar] [CrossRef]
- Moon, Y.W.; Lee, J.H.; Yoo, S.B.; Jahng, J.W. Capsaicin receptors are colocalized with sweet/bitter receptors in the taste sensing cells of circumvallate papillae. Genes Nutr. 2010, 5, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.A.; Gutierrez, R. TRP channels at the periphery of the taste and trigeminal systems, Chapter 7. In Neurobiology of TRP Channels, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2017. [Google Scholar]
- Kawashima, M.; Imura, K.; Sato, I. Topographical organization of TRPV1-immunoreactive epithelium and CGRP-immunoreactive nerve terminals in rodent tongue. Eur. J. Histochem. 2012, 56, e21. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.Y.; Wu, S.Y. Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds. J. Neurosci. 2015, 35, 12714–12724. [Google Scholar] [CrossRef] [PubMed]
- Dewis, M.L.; Phan, T.H.; Ren, Z.; Meng, X.; Cui, M.; Mummalaneni, S.; Rhyu, M.R.; DeSimone, J.A.; Lyall, V. N-geranyl cyclopropyl-carboximide modulates salty and umami taste in humans and animal models. J. Neurophysiol. 2013, 109, 1078–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Son, H.J.; Kim, Y.; Kweon, H.J.; Suh, B.C.; Lyall, V.; Rhyu, M.R. Selective activation of hTRPV1 by N-geranyl cyclopropylcarboxamide, an amiloride-insensitive salt taste enhancer. PLoS ONE 2014, 9, e89062. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Lee, B.H.; Kim, Y.H.; Song, A.Y.; Son, H.J.; Oh, S.B.; Lyall, V.; Kim, E.Y. Soy-derived glycopeptides induce inward current in TRPV1-expressing cells by whole-cell patch-clamp recording. In Proceedings of the Association for Chemoreception Sciences, Sarasota, FL, USA, 25–29 April 2007. [Google Scholar]
- Rhyu, M.-R.; Song, A.-Y.; Kim, E.-Y.; Oh, S.B.; Lee, Y.; Lim, W.C. Pharmaceutical Composition for Preventing/Treating TRPV1 Activity-Related and Inflammation-Related Diseases or Conditions Containing Maillard peptide Separated from well-Aged Traditional Soy Sauce as Active Ingredient. U.S. Patent No. 8,653,032 B2, 18 February 2014. [Google Scholar]
- Treesukosol, Y.; Lyall, V.; Heck, G.L.; DeSimone, J.A.; Spector, A.C. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1799–R1809. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.J.; Elkaddi, N.; Garcia-Blanco, A.; Spielman, A.I.; Bachmanov, A.A.; Chung, H.Y.; Ozdener, M.H. Arginyl dipeptides increase the frequency of NaCl-elicited responses via epithelial sodium channel alpha and delta subunits in cultured human fungiform taste papillae cells. Sci. Rep. 2017, 7, 7483. [Google Scholar] [CrossRef]
- Shigemura, N.; Shirosaki, S.; Ohkuri, T.; Sanematsu, K.; Islam, A.A.; Ogiwara, Y.; Kawai, M.; Yoshida, R.; Ninomiya, Y. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am. J. Clin. Nutr. 2009, 90, 764s–769s. [Google Scholar] [CrossRef]
- Shigemura, N.; Shirosaki, S.; Sanematsu, K.; Yoshida, R.; Ninomiya, Y. Genetic and molecular basis of individual differences in human umami taste perception. PLoS ONE 2009, 4, e6717. [Google Scholar] [CrossRef]



| (mM) | Stimuli | (mM) | |
|---|---|---|---|
| R | 10 KCl + 10 HEPES | NaCl | 10 KCl + 10 HEPES + 100 NaCl |
| R | 10 KCl + 10 HEPES | NaCl + Bz | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz |
| R | 10 KCl + 10 HEPES | NaCl + Bz + RTX | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz + RTX (0–0.01) |
| R | 10 KCl + 10 HEPES | NaCl + Bz + FII or sub-fractions | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz + FII or sub-fractions |
| R | 10 KCl + 10 HEPES | NaCl + SB | 10 KCl + 10 HEPES + 100 NaCl + 0.001 SB |
| R | 10 KCl + 10 HEPES | NaCl + SB + FII | 10 KCl + 10 HEPES + 100 NaCl + 0.001 SB + FII or sub-fractions) |
| R | 10 KCl + 10 HEPES | N + Bz + SB + FII | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz + 0.001 SB + FII or sub-fractions |
| R | 10 KCl | MSG + Bz + SB | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB |
| R | 10 KCl | MSG + Bz + SB + IMP | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB + 1 IMP |
| R | 10 KCl | MSG + Bz + SB + FII | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB + FII |
| R | 10 KCl | Control-1 | 300 NH4Cl |
| R | 10 KCl | Control-2 | 300 NaCl |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhyu, M.-R.; Song, A.-Y.; Kim, E.-Y.; Son, H.-J.; Kim, Y.; Mummalaneni, S.; Qian, J.; Grider, J.R.; Lyall, V. Kokumi Taste Active Peptides Modulate Salt and Umami Taste. Nutrients 2020, 12, 1198. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041198
Rhyu M-R, Song A-Y, Kim E-Y, Son H-J, Kim Y, Mummalaneni S, Qian J, Grider JR, Lyall V. Kokumi Taste Active Peptides Modulate Salt and Umami Taste. Nutrients. 2020; 12(4):1198. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041198
Chicago/Turabian StyleRhyu, Mee-Ra, Ah-Young Song, Eun-Young Kim, Hee-Jin Son, Yiseul Kim, Shobha Mummalaneni, Jie Qian, John R. Grider, and Vijay Lyall. 2020. "Kokumi Taste Active Peptides Modulate Salt and Umami Taste" Nutrients 12, no. 4: 1198. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041198





