Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and methods
2.1. Study Population
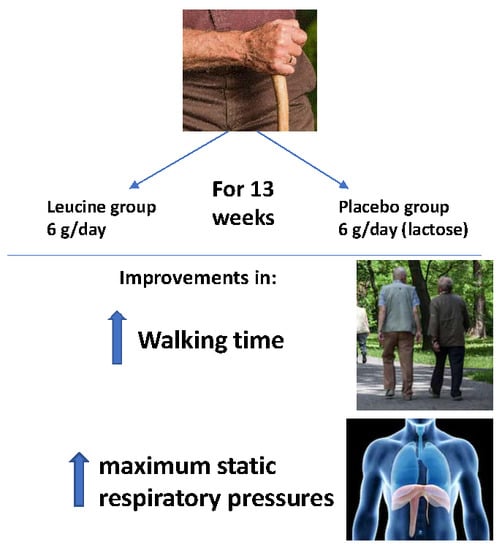
2.2. Intervention
2.3. Measurement of Sarcopenia
2.4. Geriatric Assessment
2.5. Blood Analytical Parameters
2.6. Statistical Analysis
3. Results
3.1. Design and Study Population
3.2. Dropouts, Safety and Compliance
3.3. Effect of Leucine Administration on Sarcopenia Criteria
3.4. Effect of Leucine Supplementation on Sarcopenia Muscle Respiratory Criteria
3.5. Effect of Leucine Administration on Blood Analytical Parameters
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I. The epidemiology of sarcopenia. Clin. Geriatr. Med. 2011, 27, 355–363. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Follis, S.; Cook, A.; Bea, J.W.; Going, S.B.; Laddu, D.; Cauley, J.A.; Shadyab, A.H.; Stefanick, M.L.; Chen, Z. Association between sarcopenic obesity and falls in a multiethnic cohort of postmenopausal women. J. Am. Geriatr. Soc. 2018, 66, 2314–2320. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Prince, R.L.; Scott, D.; Daly, R.M.; Duque, G.; Inderjeeth, C.A.; Zhu, K.; Woodman, R.J.; Hodgson, J.M.; Lewis, J.R. Utility of four sarcopenia criteria for the prediction of falls-related hospitalization in older Australian women. Osteoporos. Int. 2019, 30, 167–176. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014, 127, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Cuthbertson, D.J.; Bell, J.A.; Ng, S.Y.; Kemp, G.J.; Kivimaki, M.; Hamer, M. Dynapenic obesity and the risk of incident Type 2 diabetes: The English Longitudinal Study of Ageing. Diabet. Med. 2016, 33, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Dawson Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef]
- Masanés, F.; Rojano i Luque, X.; Salvà, A.; Serra-Rexach, J.A.; Artaza, I.; Formiga, F.; Cuesta, F.; López Soto, A.; Ruiz, D.; Cruz-Jentoft, A.J. Cut-off points for muscle mass—Not grip strength or gait speed—Determine variations in sarcopenia prevalence. J. Nutr. Heal. Aging 2017, 21, 825–829. [Google Scholar] [CrossRef]
- Santos, C.D.S.; Nascimento, F.E.L. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: A biochemical review. Einstein (Sao Paulo) 2019, 17, eRB4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouw, I.W.; Holwerda, A.M.; Trommelen, J.; Kramer, I.F.; Bastiaanse, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; van Loon, L.J. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J. Nutr. 2017, 147, 2252–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamarsland, H.; Nordengen, A.L.; Nyvik Aas, S.; Holte, K.; Garthe, I.; Paulsen, G.; Cotter, M.; Børsheim, E.; Benestad, H.B.; Raastad, T. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial effects of leucine supplementation on criteria for sarcopenia: A systematic review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komar, B.; Schwingshackl, L.; Hoffmann, G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: A systematic review and meta-analysis. J. Nutr. Heal. Aging 2015, 19, 437–446. [Google Scholar] [CrossRef]
- Bonnefoy, M.; Gilbert, T.; Bruyère, O.; Paillaud, E.; Raynaud-Simon, A.; Guérin, O.; Jeandel, C.; Le Sourd, B.; Haine, M.; Ferry, M.; et al. Quels bénéfices attendre de la supplémentation en protéines pour limiter la perte de masse et de fonction musculaire chez le sujet âgé fragile? Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 137–143. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [Green Version]
- ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Hickey, G.L.; Grant, S.W.; Dunning, J.; Siepe, M. Statistical primer: Sample size and power calculations-why, when and how? Eur. J. Cardio Thorac. Surg. 2018, 54, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Ezquerra, J.; Gómez Burgada, F.; Sala, J.M.; Seva Díaz, A. Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients). Actas Luso Esp. Neurol. Psiquiatr. Cienc. Afines 1979, 7, 189–202. [Google Scholar]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA®—Its history and challenges. J. Nutr. Heal. Aging 2006, 10, 456–463. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Setiati, S.; Istanti, R.; Andayani, R.; Kuswardhani, R.A.T.; Aryana, I.G.P.S.; Putu, I.D.; Apandi, M.; Ichwani, J.; Soewoto, S.; Dinda, R.; et al. Cut-off of anthropometry measurement and nutritional status among elderly outpatient in Indonesia: Multi-centre study. Acta Med. Indones. 2010, 42, 224–230. [Google Scholar] [PubMed]
- Makanae, Y.; Fujita, S. Role of Exercise and Nutrition in the Prevention of Sarcopenia. J. Nutr. Sci. Vitaminol. 2015, 61, S125–S127. [Google Scholar] [CrossRef] [Green Version]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University—Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-chain triglycerides in combination with leucine and Vitamin D increase muscle strength and function in frail elderly adults in a randomized controlled trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.S.I.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar] [CrossRef] [PubMed]
- Ispoglou, T.; White, H.; Preston, T.; McElhone, S.; McKenna, J.; Hind, K. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur. J. Clin. Nutr. 2016, 70, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Dal Negro, R.W.; Testa, A.; Aquilani, R.; Tognella, S.; Pasini, E.; Barbieri, A.; Boschi, F. Essential amino acid supplementation in patients with severe COPD: A step towards home rehabilitation. Monaldi Arch. Chest Dis. 2012, 77, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; de Vogel-van den Bosch, J.; Weijs, P.J.M. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, S.; Vanschoonbeek, K.; Verdijk, L.B.; Koopman, R.; Wodzig, W.K.W.H.; Dendale, P.; van Loon, L.J.C. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009, 89, 1468–1475. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; van Kranenburg, J.; Hartgens, F.; Wodzig, W.K.W.H.; Saris, W.H.M.; van Loon, L.J.C. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J. Nutr. 2011, 141, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Heal. 2014, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- del Campo Cervantes, J.M.; Macías Cervantes, M.H.; Monroy Torres, R. Effect of a resistance training program on sarcopenia and functionality of the older adults living in a nursing home. J. Nutr. Heal. Aging 2019, 23, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Najafi, Z.; Kooshyar, H.; Mazloom, R.; Azhari, A. The effect of fun physical activities on sarcopenia progression among elderly residents in nursing homes: A randomized controlled trial. J. Caring Sci. 2018, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Greising, S.M.; Mantilla, C.B.; Sieck, G.C. Functional impact of sarcopenia in respiratory muscles. Respir. Physiol. Neurobiol. 2016, 226, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Bone, A.E.; Hepgul, N.; Kon, S.; Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chronic Respir. Dis. 2017, 14, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Knowles, J.B.; Fairbarn, M.S.; Wiggs, B.J.; Chan-Yan, C.; Pardy, R.L. Dietary supplementation and respiratory muscle performance in patients with COPD. Chest 1988, 93, 977–983. [Google Scholar] [CrossRef]
- Borghi-Silva, A.; Baldissera, V.; Sampaio, L.M.M.; Pires-DiLorenzo, V.A.; Jamami, M.; Demonte, A.; Marchini, J.S.; Costa, D. L-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz. J. Med. Biol. Res. 2006, 39, 465–474. [Google Scholar] [CrossRef]
- Rafiq, R.; Prins, H.J.; Boersma, W.G.; Daniels, J.M.A.; den Heijer, M.; Lips, P.; de Jongh, R.T. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: A pilot trial. Int. J. COPD 2017, 12, 2583–2592. [Google Scholar] [CrossRef] [Green Version]
- Rogers, R.M.; Donahoe, M.; Costantino, J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease: A randomized control study. Am. Rev. Respir. Dis. 1992, 146, 1511–1517. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Verreschi, I.T.; Nery, L.E.; Goldstein, R.S.; Zamel, N.; Brooks, D.; Jardim, J.R. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest 1998, 114, 19–28. [Google Scholar] [CrossRef]
- De Bandt, J.-P. Leucine and mammalian target of rapamycin-dependent activation of muscle protein synthesis in aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef] [PubMed]
- Brunetta, H.S.; de Camargo, C.Q.; Nunes, E.A. Does l-leucine supplementation cause any effect on glucose homeostasis in rodent models of glucose intolerance? A systematic review. Amino Acids 2018, 50, 1663–1678. [Google Scholar] [CrossRef]
- Lollo, P.C.B.; Silva, L.B.C.; Batista, T.M.; Morato, P.N.; Moura, C.S.; Cruz, A.G.; Faria, J.A.F.; Carneiro, E.M.; Amaya-Farfan, J. Effects of whey protein and casein plus leucine on diaphragm the mTOR pathway of sedentary, trained rats. Food Res. Int. 2012, 49, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Can, B.; Kara, O.; Kizilarslanoglu, M.C.; Arik, G.; Aycicek, G.S.; Sumer, F.; Civelek, R.; Demirtas, C.; Ulger, Z. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 2017, 29, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [Green Version]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D deficiency and sarcopenia in older persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Paula, A.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2017, 175, 11. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, C.M.; Sternberg, M.R.; Schleicher, R.L.; Haynes, B.M.H.; Rybak, M.E.; Pirkle, J.L. The CDC’s second national report on biochemical indicators of diet and nutrition in the U.S. population is a valuable tool for researchers and policy makers. J. Nutr. 2013, 143, 938S–947S. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Verdijk, L.B.; de Groot, L.C.P.G.M.; van Loon, L.J.C. Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; de Oliveira Gonçalvez, I.; Sampaio, R.A.C.; Sewo Sampaio, P.Y.; Cadore, E.L.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Periodized and non-periodized resistance training programs on body composition and physical function of older women. Exp. Gerontol. 2019, 121, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Osuka, Y.; Kojima, N.; Wakaba, K.; Miyauchi, D.; Tanaka, K.; Kim, H. Effects of resistance training and/or beta-hydroxy-beta-methylbutyrate supplementation on muscle mass, muscle strength and physical performance in older women with reduced muscle mass: Protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open 2019, 9, e025723. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuku, S.; Kajioka, T.; Sakakibara, H.; Shimaoka, K. Slow movement resistance training using body weight improves muscle mass in the elderly: A randomized controlled trial. Scand. J. Med. Sci. Sports 2018, 28, 1339–1344. [Google Scholar] [CrossRef] [PubMed]


| PLACEBO Mean Values (± Standard Deviation or Percentage) | LEUCINE Mean Value (± Standard Deviation or Percentage) | p Value | |
|---|---|---|---|
| Deambulation | Independent 68.2% Walker 31.8 % | Independent 65.0% Walker 35.0% | 0.14 |
| Barthel index score | 78.0 ± 21.6 | 78.7 ± 21.2 | 0.92 |
| MMSE (Mini Mental State Examination) score | 27.9 ± 5.1 | 29.3 ± 4.0 | 0.337 |
| Comorbidities (Charlson index) | 5.4 ± 2.1 | 5.1 ± 1.7 | 0.69 |
| Nutritional status (Mini Nutritional assessment) | Normal 77.3% Malnutrition risk 22.7% | Normal 63.2% Malnutrition risk 36.8% | 0.32 |
| Body mass index (kg/m2) | 29.1 ± 5.7 | 28.9 ± 7.1 | 0.91 |
| Fat (% of body weight) | 42.9 ± 15.7 | 40.4 ± 8.9 | 0.57 |
| Fat (kg) | 29.8 ± 11.1 | 28.3 ± 10.9 | 0.69 |
| Fat mass index (Kg/m2) | 11.6 ± 5.2 | 12.0 ± 5.5 | 0.79 |
| Calf perimeter | Men: 35.3 ± 5.3 Women: 35.8 ± 5.6 | Men: 34.7 ± 5.0 Women: 33.5 ± 4.2 | p = 0.62 p = 0.24 |
| Arm perimeter | Men: 28.7 ± 4.3 Women: 29.11 ± 3.2 | Men: 29.2 ± 4.1 Women: 28.5 ± 3.5 | p = 0.46 p = 0.62 |
| Lean mass index Janssen (kg/m2) | 10.4 ± 3.4 | 8.3 ± 1.8 | 0.02 |
| Muscular handgrip strength (kg) | 19.2 ± 8.6 | 16.3 ± 8.5 | 0.28 |
| Walking time (sec) | 10.4 ± 12.5 | 10.4 ± 10.5 | 1.00 |
| Variable | PG | LG | p value |
|---|---|---|---|
| Leukocytes (× 103/µL) | 7.5 ± 0.4 | 7.5 ± 0.6 | 0.97 |
| Neutrophils (× 103/µL) | 4.4 ± 0.2 | 4.5 ± 0.2 | 0.95 |
| Lymphocytes (× 103/µL) | 2.3 ± 0.1 | 2.2 ± 0.2 | 0.94 |
| Monocytes (× 103/µL) | 0.53 ± 0.03 | 0.54 ± 0.02 | 0.98 |
| Eosinophils (× 103/µL) | 0.22 ± 0.04 | 0.22 ± 0.05 | 1.00 |
| Basophils (× 103/µL) | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.00 |
| Platelets (× 103/µL) | 240 ± 38 | 234 ± 32 | 0.82 |
| Erythrocytes (× 106/µL) | 5.0 ± 0.7 | 4.9 ± 0.4 | 0.86 |
| Hemoglobin (g/dL) | 12.3 ± 1.0 | 12.9 ± 1.2 | 0.97 |
| Glucose (mg/dL) | 97 ± 13 | 93 ± 12 | 0.88 |
| Urea (mg/dL) | 42 ± 5 | 44 ± 7 | 0.88 |
| GOT (U/L) | 28 ±4 | 27 ± 3 | 0.86 |
| GPT (U/L) | 23 ± 2 | 22 ± 4 | 0.94 |
| HDL cholesterol (mg/dL) | 44 ± 5 | 43 ± 7 | 0.96 |
| LDL cholesterol (mg/dL) | 122 ± 10 | 125 ± 14 | 0.71 |
| Triglycerides (mg/dL) | 127 ± 24 | 131 ± 18 | 0.70 |
| Total Proteins (g/dL) | 7.1 ± 0.3 | 7.2 ± 0.5 | 0.84 |
| Creatinine (mg/dL) | 0.81 ± 0.12 | 0.82 ± 0.13 | 0.91 |
| Calcium (mg/dL) | 8.5 ± 0.7 | 8.5 ± 0.8 | 1.00 |
| Sodium (mEq/L) | 140 ± 3 | 141 ± 4 | 0.91 |
| Potassium (mEq/L) | 4.5 ± 0.9 | 4.5 ± 0.7 | 0.96 |
| C-reactive Protein (mg/L) | 5.1 ± 1.8 | 5.6 ± 1.4 | 0.41 |
| TNF-α (pg/mL) | 2.8 ± 0.3 | 3.2 ± 0.5 | 0.42 |
| IL-6 (pg/mL) | 2.0 ± 0.3 | 2.4 ± 0.6 | 0.31 |
| Vit- D (25OHD) (ng/mL) | 40.1 ± 5.1 | 41.2 ± 5.2 | 0.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12040932
Martínez-Arnau FM, Fonfría-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, van Doesburg F, Pruimboom L, Fernández-Garrido J, Cauli O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients. 2020; 12(4):932. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12040932
Chicago/Turabian StyleMartínez-Arnau, Francisco M., Rosa Fonfría-Vivas, Cristina Buigues, Yolanda Castillo, Pilar Molina, Aldert J. Hoogland, Femke van Doesburg, Leo Pruimboom, Julio Fernández-Garrido, and Omar Cauli. 2020. "Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial" Nutrients 12, no. 4: 932. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12040932