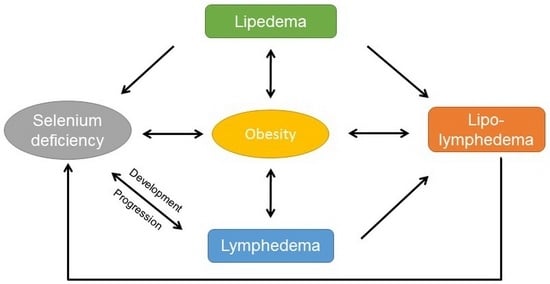
Selenium Deficiency in Lymphedema and Lipedema—A Retrospective Cross-Sectional Study from a Specialized Clinic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurement of Whole Blood Selenium
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Selenium Deficiency in the Overall Study Population
3.3. Selenium Deficiency in Lymphedema
3.4. Selenium Deficiency in Lipedema and Lipo-Lymphedema
3.5. Obesity Increases the Risk for Selenium Deficiency in Lymphedema
4. Discussion
4.1. Selenium Deficiency in the Total Study Population
4.2. Selenium Deficiency in Lymphedema
4.3. Obesity Drives Selenium Deficiency
4.4. Differences in Selenium Status Between Lipedema and Lymphedema
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hespe, G.E.; Nores, G.G.; Huang, J.-J.; Mehrara, B.J. Pathophysiology of lymphedema-Is there a chance for medication treatment? J. Surg. Oncol. 2017, 115, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Lipedema—An update. Dermatol. Ther. 2019, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Brauer, W.J.; Weissleder, H. Methodik und Ergebnisse der Funktionslymphszintigraphie: Erfahrungen bei 924 Patienten. Phlebologie 2002, 31, 118–125. [Google Scholar] [CrossRef]
- Birkballe, S.; Jensen, M.R.; Noerregaard, S.; Gottrup, F.; Karlsmark, T. Can tissue dielectric constant measurement aid in differentiating lymphoedema from lipoedema in women with swollen legs? Br. J. Dermatol. 2014, 170, 96–102. [Google Scholar] [CrossRef]
- Peprah, K.; MacDougall, D. Liposuction for the Treatment of Lipedema: A Review of Clinical Effectiveness and Guidelines; CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2019. [Google Scholar]
- Forner-Cordero, I.; Szolnoky, G.; Forner-Cordero, A.; Kemény, L. Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome—Systematic review. Clin. Obes. 2012, 2, 86–95. [Google Scholar] [CrossRef]
- Cucchi, F.; Rossmeislova, L.; Simonsen, L.; Jensen, M.R.; Bülow, J. A vicious circle in chronic lymphoedema pathophysiology? An adipocentric view. Obes. Rev. 2017, 18, 1159–1169. [Google Scholar] [CrossRef]
- Herpertz, U. Der Mißbrauch des Lipödems. LymphForsch 2003, 7, 90–93. [Google Scholar]
- Hosseini, B.; Saedisomeolia, A.; Allman-Farinelli, M. Association Between Antioxidant Intake/Status and Obesity: A Systematic Review of Observational Studies. Biol. Trace Elem. Res. 2017, 175, 287–297. [Google Scholar] [CrossRef]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum selenium determinants in French adults: The SU.VI.M.AX study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Medscape Gen. Med. 2006, 8, 59. [Google Scholar]
- Alasfar, F.; Ben-Nakhi, M.; Khoursheed, M.; Kehinde, E.O.; Alsaleh, M. Selenium is significantly depleted among morbidly obese female patients seeking bariatric surgery. Obes. Surg. 2011, 21, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Powers, S.K.; Dirks, A.J.; Scarpace, P.J. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int. J. Obes. 2001, 25, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2005, 30, 400–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [Green Version]
- Vaillant, L.; Gironet, N. Infectious complications of lymphedema. Rev. Med. Interne 2002, 23 (Suppl. 3), 403s–407s. [Google Scholar] [CrossRef]
- Kasseroller, R.G.; Schrauzer, G.N. Treatment of secondary lymphedema of the arm with physical decongestive therapy and sodium selenite: A review. Am. J. Ther. 2000, 7, 273–279. [Google Scholar] [CrossRef]
- Micke, O.; Bruns, F.; Mücke, R.; Schäfer, U.; Glatzel, M.; DeVries, A.F.; Schönekaes, K.; Kisters, K.; Büntzel, J. Selenium in the treatment of radiation-associated secondary lymphedema. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 40–49. [Google Scholar] [CrossRef]
- Bruns, F.; Büntzel, J.; Mücke, R.; Schönekaes, K.; Kisters, K.; Micke, O. Selenium in the treatment of head and neck lymphedema. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2004, 13, 185–190. [Google Scholar] [CrossRef]
- Büntzel, J.; Riesenbeck, D.; Glatzel, M.; Berndt-Skorka, R.; Riedel, T.; Mücke, R.; Kisters, K.; Schönekaes, K.G.; Schäfer, U.; Bruns, F.; et al. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. results of a randomized study in head and neck cancer patients. Anticancer Res. 2010, 30, 1829–1832. [Google Scholar]
- Greene, A.K.; Grant, F.D.; Slavin, S.A. Lower-extremity lymphedema and elevated body-mass index. N. Engl. J. Med. 2012, 366, 2136–2137. [Google Scholar] [CrossRef]
- Siems, W.G.; Brenke, R.; Beier, A.; Grune, T. Oxidative stress in chronic lymphoedema. QJM 2002, 95, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Siems, W.; Grune, T.; Voss, P.; Brenke, R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. Biofactors Oxf. Engl. 2005, 24, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tabibiazar, R.; Cheung, L.; Han, J.; Swanson, J.; Beilhack, A.; An, A.; Dadras, S.S.; Rockson, N.; Joshi, S.; Wagner, R.; et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006, 3, e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.G.; Evenson, J.K.; Thompson, K.M.; Sunde, R.A. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J. Nutr. 1995, 125, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.-P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort: Selenium status is associated with colorectal cancer risk. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef]
- Muecke, R.; Klotz, T.; Giedl, J.; Buentzel, J.; Kundt, G.; Kisters, K.; Prott, F.-J.; Micke, O. Whole blood selenium levels (WBSL) in patients with prostate cancer (PC), benign prostatic hyperplasia (BPH) and healthy male inhabitants (HMI) and prostatic tissue selenium levels (PTSL) in patients with PC and BPH. Acta Oncol. 2009, 48, 452–456. [Google Scholar] [CrossRef]
- Anke, M.; Glei, M.; Dorn, W.; Müller, R.; Vormann, J.; Müller, M.; Jahritz, M.; Seifert, M.; Holzinger, S.; Drobner, S.; et al. Trace Element Intake and Balance in Adults in Central Europe. In Trace Elements in Man and Animals 10; Roussel, A.M., Anderson, R.A., Favrier, A.E., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 209–214. ISBN 978-0-306-46378-5. [Google Scholar]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- S2k Guideline—Diagnostics and Therapy of Lymphoedema. 2017. Available online: https://www.awmf.org/fileadmin/user_upload/Leitlinien/058_Ges_D_Lymphologen/058-001le_S2k_Diagnostics_and_therapy_of_lymphoedema_2019-07.pdf (accessed on 24 April 2020).
- Winnefeld, K.; Dawczynski, H.; Schirrmeister, W.; Adam, G.; Friedrich, U.; Hein, S. Selenium in serum and whole blood in patients with surgical interventions. Biol. Trace Elem. Res. 1995, 50, 149–155. [Google Scholar] [CrossRef]
- Fachinformation Selenase® T Peroral, Biosyn Arzneimittel GmbH, Stand Nov. 2017. Available online: https://www.gelbe-liste.de/produkte/selenase-T-peroral_354493/fachinformation (accessed on 24 April 2020).
- Muecke, R.; Waldschock, K.; Schomburg, L.; Micke, O.; Buentzel, J.; Kisters, K.; Adamietz, I.A.; Huebner, J. Whole Blood Selenium Levels and Selenium Supplementation in Patients Treated in a Family Doctor Practice in Golßen (State of Brandenburg, Germany): A Laboratory Study. Integr. Cancer Ther. 2018, 17, 1132–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobedo, N.; Oliver, G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab. 2017, 26, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, Y.; Reinhart, K.; Bloos, F.; Marx, G.; Russwurm, S.; Bauer, M.; Brunkhorst, F. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br. J. Anaesth. 2007, 98, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2017. [Google Scholar] [CrossRef]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P status correlates to cancer-specific mortality in renal cancer patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.; Waters, R.; Sieniawska, C.; Kassam, S.; Montoto, S.; Fitzgibbon, J.; Rohatiner, A.; Lister, A.; Joel, S. Serum selenium concentration at diagnosis and outcome in patients with haematological malignancies. Br. J. Haematol. 2011, 154, 448–456. [Google Scholar] [CrossRef]
- Franca, C.A.S.; Nogueira, C.R.; Ramalho, A.; Carvalho, A.C.P.; Vieira, S.L.; Penna, A.B.R.C. Serum levels of selenium in patients with breast cancer before and after treatment of external beam radiotherapy. Ann. Oncol. 2011, 22, 1109–1112. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Xue, M.; Chi, F.; Xu, Z.G.; Fan, G.L.; Fan, Y.C.; Zheng, M.H.; Zhong, W.Z.; Wang, S.L.; Zhang, Z.Y.; et al. Serum levels of selenium in patients with brain metastases from non-small cell lung cancer before and after radiotherapy. Cancer Radiothér. 2012, 16, 179–182. [Google Scholar] [CrossRef]
- Charalabopoulos, K.; Kotsalos, A.; Batistatou, A.; Charalabopoulos, A.; Peschos, D.; Vezyraki, P.; Kalfakakou, V.; Metsios, A.; Charalampopoulos, A.; Macheras, A.; et al. Serum and tissue selenium levels in gastric cancer patients and correlation with CEA. Anticancer Res. 2009, 29, 3465–3467. [Google Scholar] [PubMed]
- Kim, I.-W.; Bae, S.-M.; Kim, Y.-W.; Liu, H.-B.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chaturvedi, P.K.; Battogtokh, G.; Ahn, W.S. Serum selenium levels in Korean hepatoma patients. Biol. Trace Elem. Res. 2012, 148, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bruns, F.; Micke, O.; Bremer, M. Current status of selenium and other treatments for secondary lymphedema. J. Support. Oncol. 2003, 1, 121–130. [Google Scholar] [PubMed]
- Zimmermann, T.; Leonhardt, H.; Kersting, S.; Albrecht, S.; Range, U.; Eckelt, U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol. Trace Elem. Res. 2005, 106, 193–203. [Google Scholar] [CrossRef]
- Pfister, C.; Dawzcynski, H.; Schingale, F.-J. Sodium selenite and cancer related lymphedema: Biological and pharmacological effects. J. Trace Elem. Med. Biol. 2016, 37, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Han, H.W.; Yang, E.J.; Lee, S.-M. Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System. Nutrients 2019, 11, 1021. [Google Scholar] [CrossRef] [Green Version]
- Angstwurm, M.W.A.; Engelmann, L.; Zimmermann, T.; Lehmann, C.; Spes, C.H.; Abel, P.; Strauss, R.; Meier-Hellmann, A.; Insel, R.; Radke, J.; et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit. Care Med. 2007, 35, 118–126. [Google Scholar] [CrossRef]
- Andrews, P.J.D.; Avenell, A.; Noble, D.W.; Campbell, M.K.; Croal, B.L.; Simpson, W.G.; Vale, L.D.; Battison, C.G.; Jenkinson, D.J.; Cook, J.A.; et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011, 342, d1542. [Google Scholar] [CrossRef] [Green Version]
- Valenta, J.; Brodska, H.; Drabek, T.; Hendl, J.; Kazda, A. High-dose selenium substitution in sepsis: A prospective randomized clinical trial. Intensive Care Med. 2011, 37, 808–815. [Google Scholar] [CrossRef]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients with Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern. Med. 2016. [Google Scholar] [CrossRef]
- Chelkeba, L.; Ahmadi, A.; Abdollahi, M.; Najafi, A.; Ghadimi, M.; Mosaed, R.; Mojtahedzadeh, M. The effect of high-dose parenteral sodium selenite in critically ill patients following sepsis: A clinical and mechanistic study. Indian J. Crit. Care Med. 2017, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; García Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary Selenium Modulates Activation and Differentiation of CD4+ T Cells in Mice through a Mechanism Involving Cellular Free Thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef]
- Contempre, B.; Le Moine, O.; Dumont, J.E.; Denef, J.F.; Many, M.C. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta). Mol. Cell. Endocrinol. 1996, 124, 7–15. [Google Scholar] [CrossRef]
- Oglio, R.; Thomasz, L.; Salvarredi, L.; Juvenal, G.; Pisarev, M. Comparative effects of transforming growth factor beta isoforms on redox metabolism in thyroid cells. Mol. Cell. Endocrinol. 2018, 470, 168–178. [Google Scholar] [CrossRef]
- Azab, S.F.; Saleh, S.H.; Elsaeed, W.F.; Elshafie, M.A.; Sherief, L.M.; Esh, A.M. Serum trace elements in obese Egyptian children: A case–control study. Ital. J. Pediatr. 2014, 40, 20. [Google Scholar] [CrossRef] [Green Version]
- Błażewicz, A.; Klatka, M.; Astel, A.; Korona-Glowniak, I.; Dolliver, W.; Szwerc, W.; Kocjan, R. Serum and urinary selenium levels in obese children: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 29, 116–122. [Google Scholar] [CrossRef]
- Chang, T.-C.; Uen, Y.-H.; Chou, C.-H.; Sheu, J.-R.; Chou, D.-S. The role of cyclooxygenase-derived oxidative stress in surgically induced lymphedema in a mouse tail model. Pharm. Biol. 2013, 51, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Shavit, E.; Wollina, U.; Alavi, A. Lipoedema is not lymphoedema: A review of current literature. Int. Wound J. 2018, 15, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Mondry TE, N.S. Bucher’s Broom and Selenium Improve Lipedema: A Retrospective Case Study. Altern. Integr. Med. 2013, 2, 119. [Google Scholar] [CrossRef] [Green Version]




| Characteristic | n = (%) * |
|---|---|
| Sample, n | 791 |
| Primary lymphedema | 78 |
| Secondary lymphedema, total | 347 |
| Non-cancer | 201 |
| Cancer | 146 |
| Lipedema | 198 |
| Lipo-lymphedema | 168 |
| Selenium status †, μg/L, mean (SD) | 100.6 ± 17.4 |
| Selenium deficiency | 376 (47.5) |
| Characteristic | Selenium Concentration in Whole Blood *, μg/L, Mean (SD) | |
|---|---|---|
| Primary Lymphedema | Secondary Lymphedema | |
| Sample, n | 78 | 347 |
| All | 104.0 ± 20.9 | 100.5 ± 20.3 |
| Selenium deficiency, n (%) | 34 (43.6) | 170 (49.0) |
| BMI < 30 | 108.8 ± 22.2 | 103.3 ± 19.5 |
| BMI ≥ 30 < 40 | 97.2 ± 18.2 | 99.4 ± 22.9 |
| BMI ≥ 40 | 99.9 ± 15.4 | 96.0 ± 19.9 |
| p trend † | 0.0646 | 0.0041 |
| Characteristic | Selenium Concentration in Whole Blood *, μg/L, Mean (SD) | ||
|---|---|---|---|
| Lipedema | Lipo-Lymphedema | p Value † | |
| Sample, n | 198 | 168 | |
| All | 101.7 ± 12.3 | 98.4 ± 15.6 | 0.0127 |
| Selenium deficiency, n (%) | 83 (41.9) | 89 (53.0) | 0.0347 |
| BMI < 30 | 101.7 ± 14.2 | 103.3 ± 13.3 | 0.7343 |
| BMI ≥ 30 < 40 | 101.8 ± 11.1 | 97.7 ± 18.9 | 0.0692 |
| BMI ≥ 40 | 101.5 ± 14.0 | 96.4 ± 14.4 | 0.0254 |
| Odds Ratio (95 % CI) | p Value † | |
|---|---|---|
| Total | ||
| BMI < 30 | Ref. | Ref. |
| BMI ≥ 30 | 1.73 (1.30 to 2.30) | 0.0002 |
| BMI ≥ 40 | 1.76 (1.27 to 2.43) | 0.0006 |
| Lymphedema | ||
| BMI < 30 | Ref. | Ref. |
| BMI ≥ 30 | 2.19 (1.49 to 3.21) | <0.0001 |
| BMI ≥ 40 | 2.37 (1.49 to 3.74) | 0.0002 |
| Lipo-Lymphedema | ||
| BMI < 30 | Ref. | Ref. |
| BMI ≥ 30 | 1.85 (0.90 to 3.71) | 0.0894 |
| BMI ≥ 40 | 1.88 (0.91 to 4.11) | 0.0995 |
| Lipedema | ||
| BMI < 30 | Ref. | Ref. |
| BMI ≥ 30 | 0.95 (0.46 to 1.93) | 0.8811 |
| BMI ≥ 40 | 1.11 (0.58 to 2.11) | 0.7504 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfister, C.; Dawczynski, H.; Schingale, F.-J. Selenium Deficiency in Lymphedema and Lipedema—A Retrospective Cross-Sectional Study from a Specialized Clinic. Nutrients 2020, 12, 1211. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051211
Pfister C, Dawczynski H, Schingale F-J. Selenium Deficiency in Lymphedema and Lipedema—A Retrospective Cross-Sectional Study from a Specialized Clinic. Nutrients. 2020; 12(5):1211. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051211
Chicago/Turabian StylePfister, Christina, Horst Dawczynski, and Franz-Josef Schingale. 2020. "Selenium Deficiency in Lymphedema and Lipedema—A Retrospective Cross-Sectional Study from a Specialized Clinic" Nutrients 12, no. 5: 1211. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051211