Anti-Obesity Effects of Petasites japonicus (Meowi) Ethanol Extract on RAW 264.7 Macrophages and 3T3-L1 Adipocytes and Its Characterization of Polyphenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Reagents
2.2. Cell Culture and Viability
2.3. Measurement of Nitric Oxide
2.4. Oil-red O Staining
2.5. Measurement of Chemokine and Adipocytokines
2.6. Analysis of mRNA Expression
2.7. Extraction of Phenolic Acids and Flavonoids
2.8. UPLC-DAD-QTOF/MS Conditions
2.9. Statistical Analysis
3. Results
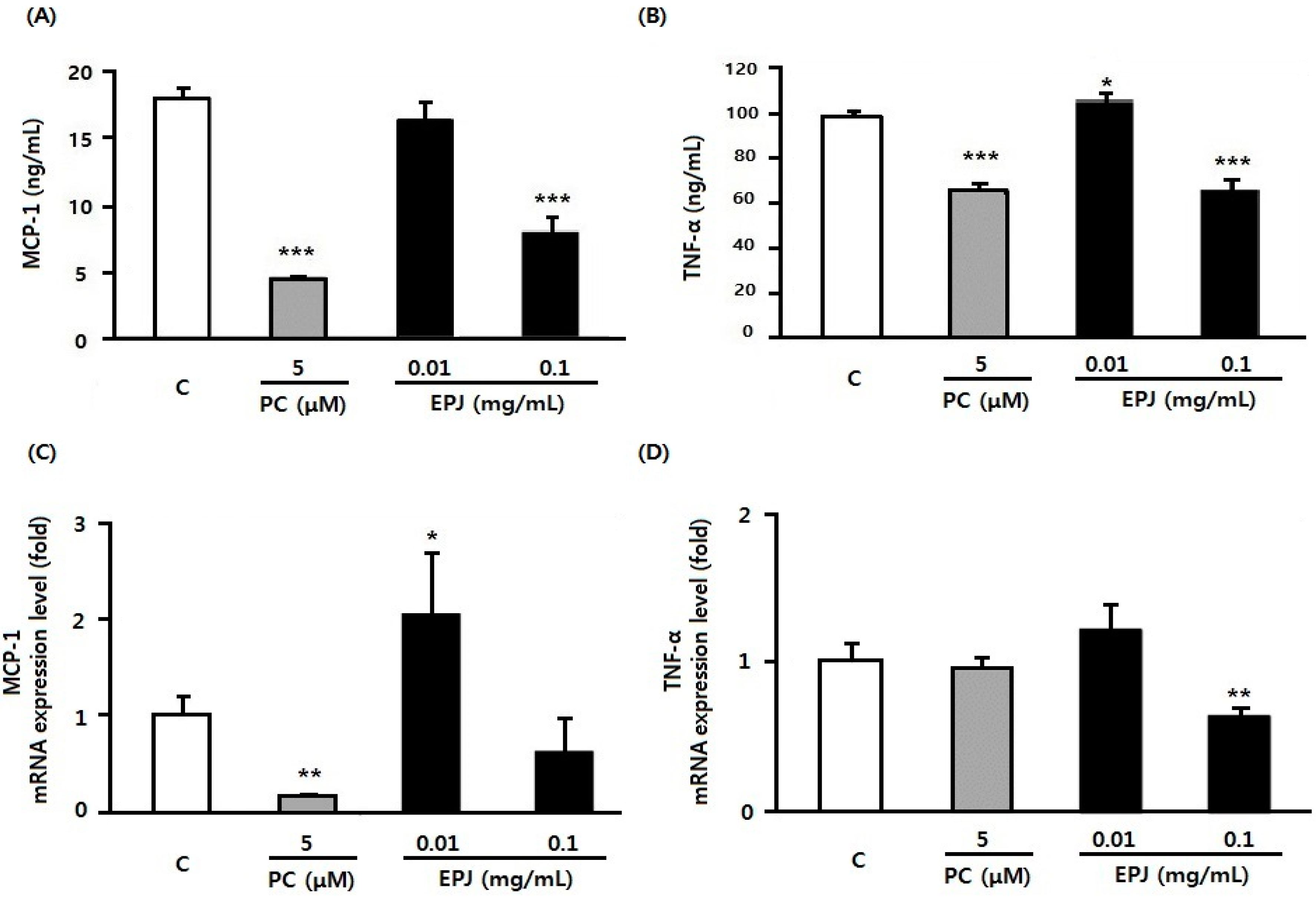
3.1. EPJ Inhibits NO Production in LPS-Stimulated Macrophages
3.2. EPJ Inhibits MCP-1 and TNFα in LPS-Stimulated Macrophages
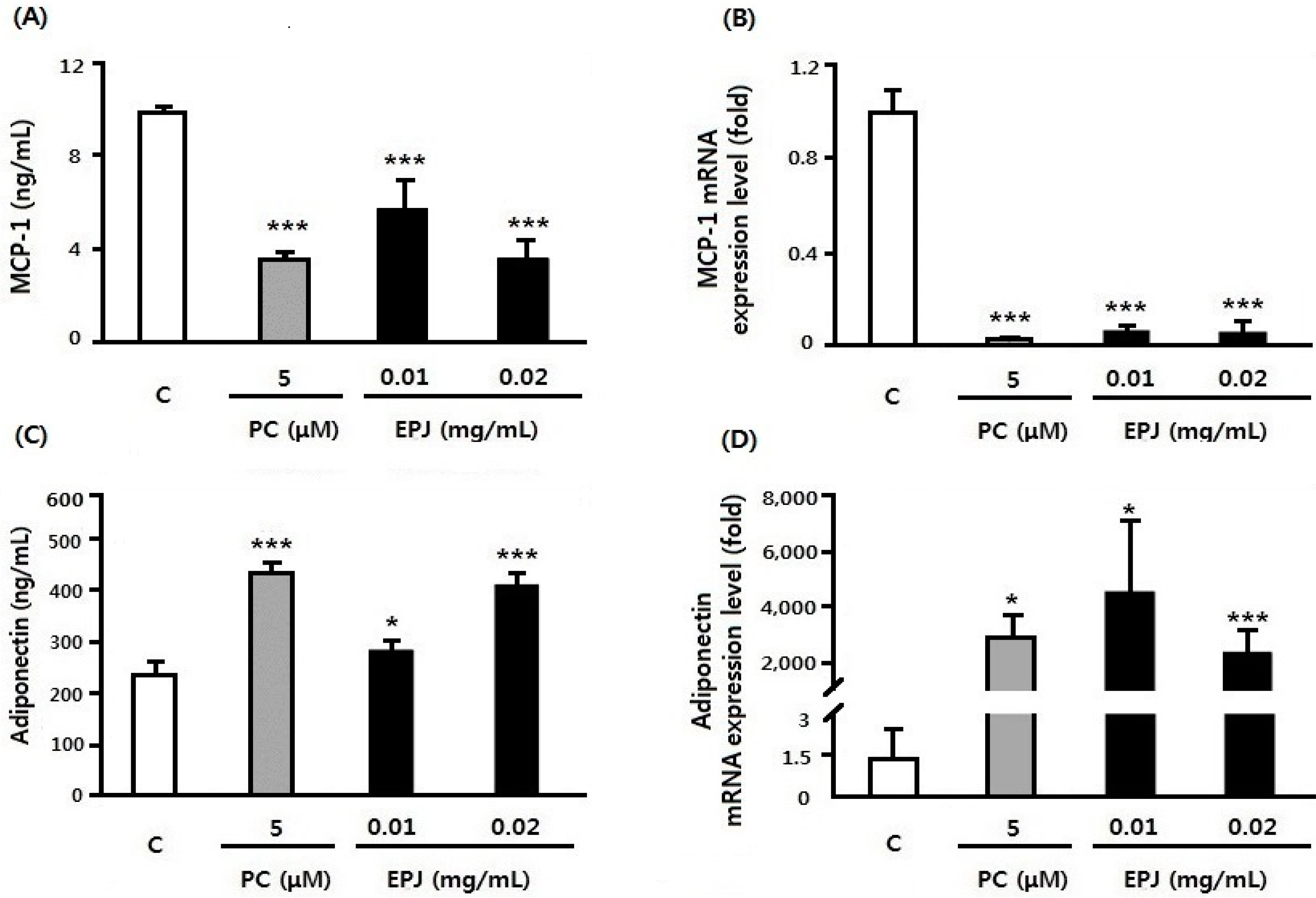
3.3. EPJ Improves MCP-1 and Adiponectin in Differentiated 3T3-L1 Adipocytes
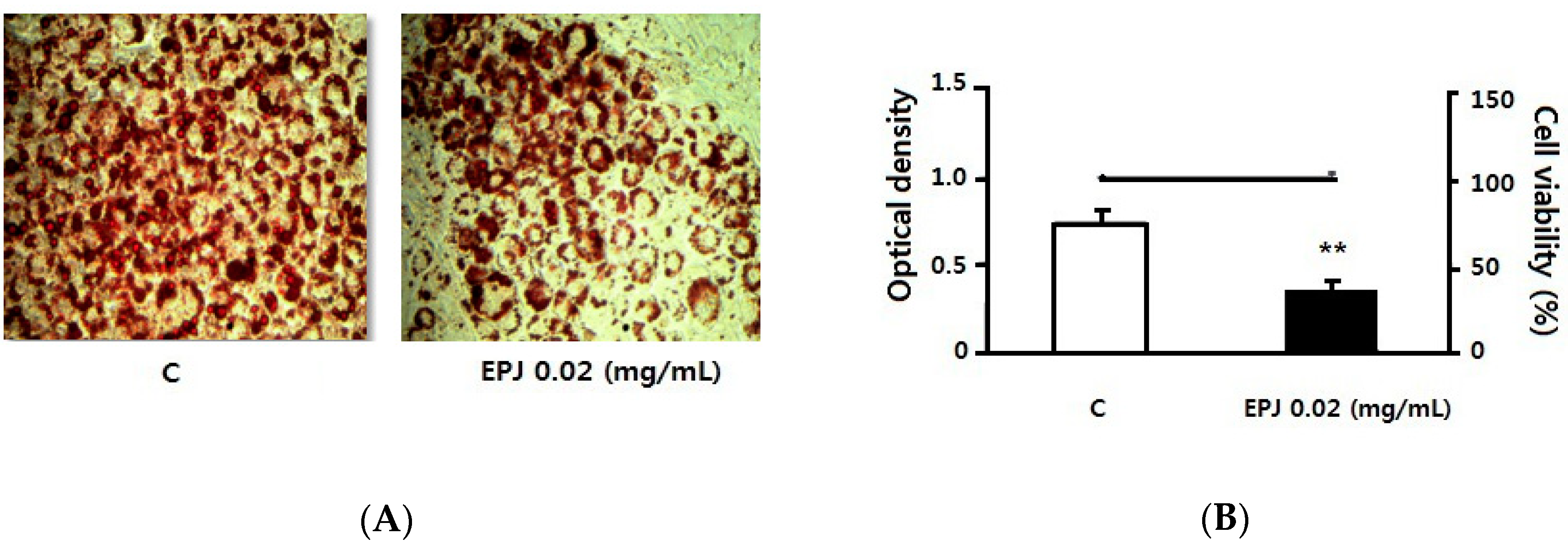
3.4. EPJ Decreases Lipid Accumulation in Differentiated 3T3-L1 Adipocytes
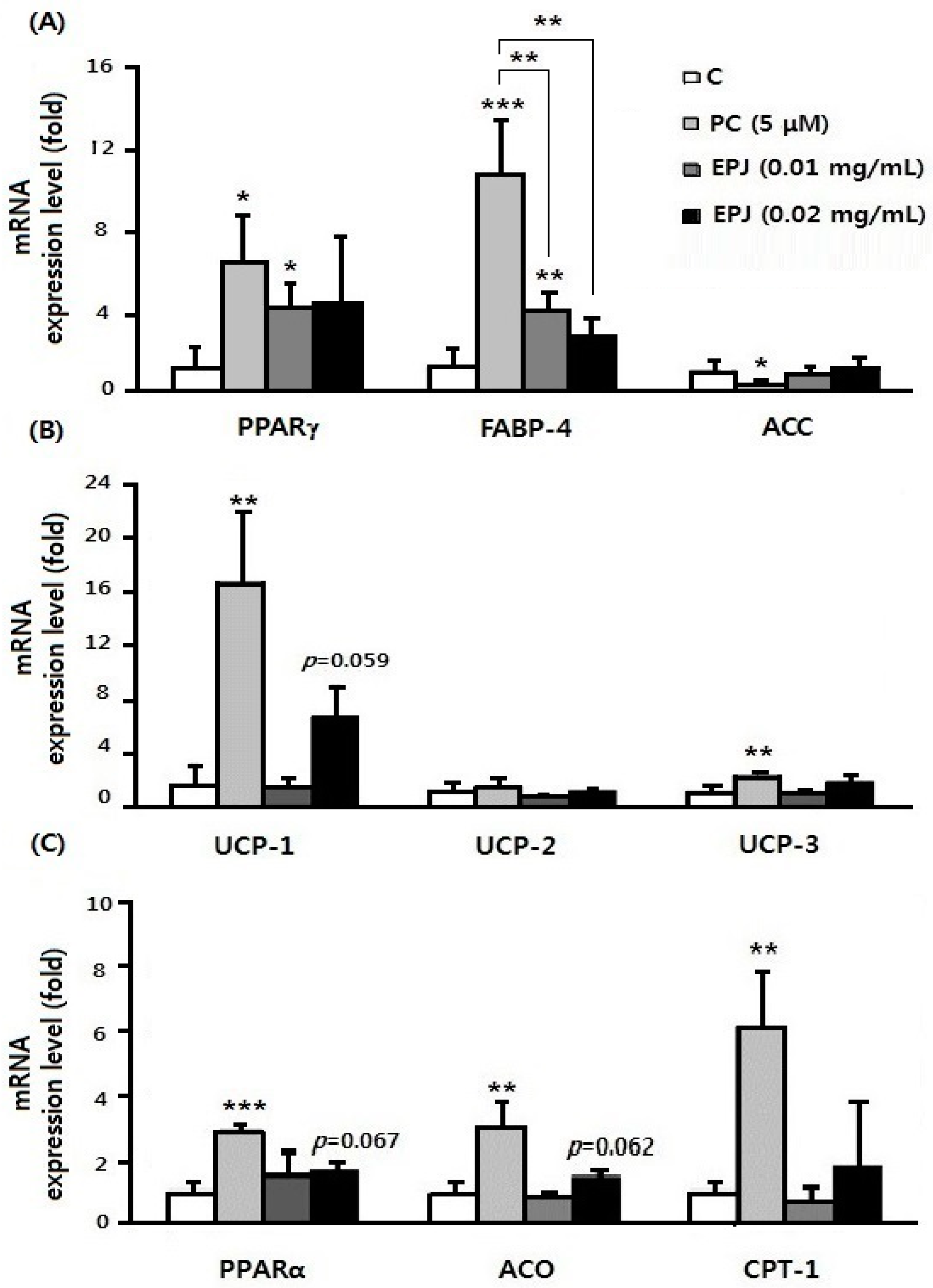
3.5. Anti-Lipogenic Effect of EPJ in Differentiated 3T3-L1 Adipocytes
3.6. Characterization of Phenolic Acids and Flavonoids in EPJ
3.6.1. Characterization of Phenolic Acids
3.6.2. Characterization of Flavonoids
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yip, W.C.Y.; Sequeira, I.R.; Plank, L.D.; Poppitt, S.D. Prevalence of pre-diabetes across ethnicities: A review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycemia. Nutrients 2017, 9, 1273. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Zhao, S.; Yu, B.; Wang, X.; Matyal, R.; Li, Y.; Jiang, Z. High-density Lipoprotein Increases the Uptake of Oxidized Low Density Lipoprotein via PPARgamma/CD36 Pathway in Inflammatory Adipocytes. Int. J. Biol. Sci. 2015, 11, 256–265. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Kawada, T.; Goto, T.; Hirai, S.; Kang, M.S.; Uemura, T.; Yu, R.; Takahashi, N. Dietary regulation of nuclear receptors in obesity-related metabolic syndrome. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 126–130. [Google Scholar]
- Romacho, T.; Glosse, P.; Richter, I.; Elsen, M.; Schoemaker, M.H.; van Tol, E.A.; Eckel, J. Nutritional ingredients modulate adipokine secretion and inflammation in human primary adipocytes. Nutrients 2015, 7, 865–886. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Díez, J.J.; Lglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Ukkola, O.; Santaniemi, M. Adiponection: A link between excess adiposity and associated comorbidities? J. Mol. Med. 2002, 80, 696–702. [Google Scholar] [CrossRef]
- Nedvídková, J.; Smitka, K.; Kopský, V.; Hainer, V. Adiponectin, an adipocyte-derived protein. Physiol. Res. 2005, 54, 133–140. [Google Scholar]
- Ota, T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013, 37, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsanis, C.; Zacharioudaki, V.; Androulidaki, A.; Dermitzaki, E.; Charalampopoulos, I.; Minas, V.; Gravanis, A.; Margioris, A.N. Adiponectin induces TNF alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem. Biophys. Res. Commun. 2005, 335, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ge, K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.M.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturk, Z.A.; Kadayifci, A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 199–206. [Google Scholar] [CrossRef]
- Kim, J.; Nam, H.; Kim, Y.; Jung, H.; Choi, Y.; Oh, J.; Chun, S.; Suh, J. Anti-adipogenic effects in 3T3-L1 cells of acetone extracts and fractions from Styrax japonica fruit. Food Sci. Biotechnol. 2015, 24, 1513–1521. [Google Scholar] [CrossRef]
- Moon, H.K.; Kim, K.S.; Chung, S.K.; Kim, J.K. Effect of wild Korean ginseng (Panax ginseng) extract on blood glucose and serum lipid contents in rats with multiple low-dose streptozotocin-induced diabetes. Food Sci. Biotechnol. 2015, 24, 1505–1511. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, N.; Zhang, Z.; Li, W.; Zhu, W. Resveratrol induces cell apoptosis in adipocytes via AMPK activation. Biochem. Biophys. Res. Commun. 2015, 457, 608–613. [Google Scholar] [CrossRef]
- Hassan, R.; Mohammad, H.F.; Reza, K. Polyphenols and their benefits. Int. J. Food Properies 2017, 20, 1700–1741. [Google Scholar]
- Kang, H.G.; Jeong, S.H.; Cho, J.H. Antimutagenic and anticarcinogenic effect of methanol extracts of Petasites japonicus Maxim leaves. J. Vet. Sci. 2010, 11, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Kang, S.; Park, S.J.; Choi, Y.W.; Lee, Y.G.; Im, D.S. Anti-allergic and anti-inflammatory effects of bakkenolide B isolated from Petasites japonicus leaves. J. Ethnopharmacol. 2013, 148, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yang, E.J.; Yun, C.Y.; Kim, D.H.; Kim, I.S. Suppressive effect of Petasites japonicus extract on ovalbumin-induced airway inflammation in an asthmatic mouse model. J. Ethnopharmacol. 2011, 133, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.E.; Oh, S.H.; Kim, Y.B.; Kang, H.G.; Kim, M.R. Neuroprotection by extract of Petasites japonicus leaves, a traditional vegetable, against oxidative stress in brain of mice challenged with kainic acid. Eur. J. Nutr. 2006, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Student, A.K.; Hsu, R.Y.; Lane, M.D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 1980, 255, 4745–4750. [Google Scholar]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Kuroyanagi, K.; Kang, M.S.; Goto, T.; Hirai, S.; Ohyama, K.; Kusudo, T.; Yu, R.; Yano, M.; Sasaki, T.; Takahashi, N.; et al. Citrus auraptene acts as an agonist for PPARs and enhances adiponectin production and MCP-1 reduction in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2008, 366, 219–225. [Google Scholar] [CrossRef]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Sartopy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn analysis of the cis- isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green robusta coffee beans by LC-MSn: Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Wang, H.; Lan, Y.; Chen, S. Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. leaves by HPLC-DAD-ESI-IT-TOF-MS and HPLC-DAD. J. Pharm. Biomed. Anal. 2013, 85, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Desta, K.T.; Saralamma, V.V.G.; Lee, S.J.; Lee, S.J.; Kim, S.M.; Paramanantham, A.; Lee, H.J.; Kim, Y.H.; Shin, H.C.; et al. LC-MS/MS characterization, anti-inflammatory effects and antioxidant activtiies of polyphenols from different tissues of Korean Petasities japonicus (Meowi). Biomed. Chromatogr. 2017, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar]
- Chuluyan, H.E.; Lang, B.J.; Yoshimura, T.; Kenney, J.S.; Issekutz, A.C. Chemokine production and adhesion molecule expression by neural cells exposed to IL-1, TNF alpha and interferon gamma. Life Sci. 1998, 63, 1939–1952. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes care 2016, 39, S244–S252. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Lee, J.Y.; Teraminami, A.; Kim, Y.I.; Hirai, S.; Uemura, T.; Inoue, H.; Takahashi, N.; Kawada, T. Activation of proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J. Lipid Res. 2011, 52, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Hata, K.; Hiwatashi, K.; Hori, K.; Suzuki, N.; Itoh, H. Suppression of murine preadipocyte differentiation and reduction of visceral fat accumulation by a Petasites japonicus ethanol extract in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [Green Version]
- Asano, H.; Kanamori, Y.; Higurashi, S.; Nara, T.; Kato, K.; Matsui, T.; Funaba, M. Induction of beige-like adipocytes in 3T3-L1 cells. J. Vet. Med Sci. 2014, 76, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.J.; Vicario, P.P.; Thompson, G.M.; Candelore, M.R.; Doebber, T.W.; Ventre, J.; Wu, M.S.; Meurer, R.; Forrest, M.J.; Conner, M.W.; et al. Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 1998, 139, 4920–4927. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Yoon, M.J.; Lee, G.Y.; Chung, J.J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, H.; Tanaka, J.; Yamada, E.; Moorikawa, T.; Kasajima, N.; Yoshikawa, M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J. Agric. Food Chem. 2006, 54, 2915–2920. [Google Scholar] [CrossRef]
- Watanabe, S.; Hasihmoto, K.; Tazaki, H.; Iwamoto, Y.; Shinohara, N.; Satoh, K.; Sakagami, H. Radical scavanging activity and inhibition of macrophage NO production by fukinolic acid, a main phenolic contituent in Japanese butterbur (Petasites japonicus). Food Sci. Technol. Res. 2007, 13, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Sakumura, S.; Yoshihara, T.; Toyoda, K. The constituents of Petasites japonicus: Structures of fukiic acid and fukinolic acid. Agricuural Biol. Chem. 1973, 37, 1915–1921. [Google Scholar] [CrossRef]


| Peak no. | Compound Name | RT (min) | DAD λmax (nm) | Formula [M+H]+ | ESI(+)-QToF/MS (Experimental Ions, m/z) | Content (mg/ 100 g DW) | |
|---|---|---|---|---|---|---|---|
| Exp. Mass [M+H]+ | Adducts and Fragmentation (m/z) | ||||||
| Phenolic acid | |||||||
| 2 b | caffeic acid | 16.63 | 240sh,295sh,323 | C9H9O4 | 181.0727 | 163[M+H-H2O]+, 145[M+H-2H2O]+, 135[M+H-H2O-CO]+ | 24.4 ± 0.3 |
| 1b | 3-O-caffeoylquinic acid (neochlorogenic acid) | 10.73 | 240sh,295sh,324 | C16H19O9 | 355.0504 | 393[M+K]+, 377[M+Na]+, 337[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 10.5 ± 0.2 |
| 3b | 5-O-caffeoylquinic acid (chlorogenic acid) | 17.65 | 242sh,299sh,325 | C16H19O9 | 355.0504 | 393[M+K]+, 377[M+Na]+, 337[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 299.5 ± 20.3 |
| 4b | 4-O-caffeoylquinic acid (cryptochlorogenic acid) | 20.48 | 241sh,299sh,324 | C16H19O9 | 355.0504 | 393[M+K]+, 377[M+Na]+, 337[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135 [Caf+H-H2O-CO]+ | 2.1 ± 0.1 |
| 5a | cis-5-O-caffeoylquinic acid (cis-chlorogenic acid) | 24.04 | 232,314 | C16H19O9 | 355.0504 | 393[M+K]+, 377[M+Na]+, 337[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 6.1 ± 0.1 |
| 6b | 5-O-feruloylquinic acid | 28.96 | 234,296sh,325 | C17H21O9 | 369.0629 | 407[M+K]+, 391[M+Na]+, 351[M+H-H2O]+, 195[Fr+H]+, 177[Fr+H-H2O]+, 149[Fr+H-H2O-CO]+, 145[Fr+H-H2O-CH3OH]+, 134[Fr+H-H2O-CO-CH3]+ | 3.8 ± 0.1 |
| 7a,b | 5-O-caffeoylquinic acid methyl ester | 35.28 | 244 sh,296sh,326 | C17H21O9 | 369.0629 | 407[M+K]+, 391[M+Na]+, 351[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O ]+, 135[Caf+H-H2O-CO]+ | 3.2 ± 0.2 |
| 8 | fukinolic acid | 49.23 | 226 sh,288sh,329 | C20H19O11 | 435.0651 | 473[M+K]+, 457[M+Na]+, 417[M+H-H2O]+, 181[Caf+H]+, 163[Caf+H- H2O ]+, 145[Caf+H-2H2O ]+, 135[Caf+H-H2O-CO]+ | 235.0 ± 4.8 |
| 9b | 3,4-di-O-caffeoylquinic acid (isochlorogenic acid B) | 52.42 | 241sh,297sh,324 | C25H25O12 | 517.0995 | 555[M+K]+, 539[M+Na]+, 499[M+H-H2O]+, 355[M+H-Caf]+, 337[M+H-Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 60.5 ± 1.5 |
| 10b | 3,5-di-O-caffeoylquinic acid (isochlorogenic acid A) | 53.53 | 241sh,299sh,326 | C25H25O12 | 517.0995 | 555[M+K]+, 539[M+Na]+, 499[M+H-H2O]+, 355[M+H-Caf]+, 337[M+H-Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 870.1 ± 14.5 |
| 11a,b | 1,5-di-O-caffeoylquinic acid (isochlorogenic acid A) | 54.49 | 241sh,298sh,325 | C25H25O12 | 517.0995 | 555[M+K]+, 539[M+Na]+, 499[M+H-H2O]+, 355[M+H-Caf]+, 337[M+H-Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 12.2 ± 0.3 |
| 12a | 3-O-cis-caffeoyl-5-O-caffeoylquinic acid (cis-isochlorogenic acid A) | 57.38 | 243sh,296sh,316 | C25H25O12 | 517.0995 | 555[M+K]+, 539[M+Na]+, 499[M+H-H2O]+, 355[M+H-Caf]+, 337[M+H-Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 5.4 ± 0.4 |
| 13b | 4,5-di-O-caffeoylquinic acid (isochlorogenic acid C) | 60.69 | 242sh,299sh,326 | C25H25O12 | 517.0995 | 555[M+K]+, 539[M+Na]+, 499[M+H-H2O]+, 355[M+H-Caf]+, 337[M+H-Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 77.8 ± 1.3 |
| 14a,b | 3,4,5-di-O-caffeoylquinic acid | 75.59 | 243,297sh,326 | C34H31O15 | 679.1178 | 717[M+K]+, 701[M+Na]+, 661[M+H-H2O]+, 517[M+H-Caf]+, 499[M+H-Caf-H2O]+, 337[M+H-2Caf-H2O]+, 181[Caf+H]+, 163[Caf+H-H2O]+, 145[Caf+H-2H2O]+, 135[Caf+H-H2O-CO]+ | 65.8 ± 0.4 |
| Total Phenolic acid content | 1676.4 ± 42.0 | ||||||
| Flavonoids | |||||||
| 15a | quercetin 3-O-(6″-O-acetyl) glucoside-7-O-glucoside | 10.11 | 255,268sh, 301sh 345 | C29H33O18 | 669.1531 | 691[M+Na] +, 465[M+H-Ac-Glu] +, 303[M+H-Ac-2Glu] + | 1.0 ± 0.1 |
| 16a | kaempferol 3-O-(6″-O-acetyl) glucoside-7-O-glucoside | 11.97 | 263,331 | C29H33O17 | 653.1549 | 675[M+Na]+, 491[M+H-Glu]+, 449[M+H-Ac-Glu]+, 287[M+H-Ac-2Glu]+ | 11.2 ± 1.2 |
| 17a | quercetin 3-O-rutinoside (rutin) | 14.06 | 257,265sh, 297sh, 354 | C27H31O16 | 611.1428 | 633[M+Na] +, 465[M+H-Rham] +, 303[M+H-Rut] + | 3.6 ± 0.5 |
| 18 | quercetin 3-O-glucoside (isoquercitrin) | 14.57 | 257,265sh, 298sh,356 | C21H21O12 | 465.0908 | 487[M+Na] +, 303[M+H-Glu] + | 36.3 ± 4.2 |
| 19a | quercetin 3-O-(6″-O-malonyl) glucoside | 15.83 | 238,291sh, 335 | C24H23O15 | 551.0884 | 573[M+Na] +, 303[M+H-Mal-Glu] + | 18.9 ± 2.2 |
| 20a | kaempferol 3-O-rutinoside (nicotiflorin) | 15.94 | 266,347 | C27H31O15 | 595.1497 | 617[M+Na] +, 449[M+H-Rham] +, 287[M+H-Rut] + | 112.1 ± 13.2 |
| 21 | kaempferol 3-O-glucoside (astragalin) | 16.57 | 269,342 | C21H21O11 | 449.0971 | 471[M+Na]+, 287[M+H-Glu] + | 132.6 ± 15.7 |
| 22 | quercetin 3-O-(6″-O-acetyl) glucoside | 17.13 | 257,266sh,296sh, 355 | C23H23O13 | 507.1000 | 529[M+Na] +, 303[M+H-Ac-Glu] + | 140.9 ± 16.4 |
| 23a | kaempferol 3-O-(6″-O-malonyl) glucoside | 18.30 | 266,298sh,351 | C27H31O15 | 535.0933 | 557[M+Na] +, 287[M+H-Mal-Glu] + | 44.9 ± 5.3 |
| 24a | quercetin 3-O-(6″-O-caffeoyl) glucoside | 18.83 | 254,267sh,301sh, 334 | C30H27O15 | 627.1175 | 649[M+Na] +, 303[M+H-Caf-Glu] +, 181[Caf+H] +, 163[Caf+H-H2O] +, 145[Caf+H-2H2O] +, 135[Caf+H-H2O-CO] + | 50.8 ± 6.1 |
| 25 | kaempferol 3-O-(6″-O-acetyl) glucoside | 19.78 | 266,300sh,348 | C23H23O12 | 491.1042 | 513[M+Na] +, 287[M+H-Ac-Glu] + | 429.6 ± 51.4 |
| 26a | kaempferol 3-O-(6″-O-caffeoyl) glucoside | 21.07 | 267,330 | C27H31O14 | 611.1226 | 633[M+Na]+, 287[M+H-Caf-Glu]+, 181[Caf+H]+, 163[Caf+H-H2O] +, 145[Caf+H-2H2O] +, 135[Caf+H- H2O-CO] + | 82.9 ± 10.8 |
| Total flavonoid content | 1064.8 ± 124.6 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, E.M.; Asamenew, G.; Kim, H.W.; Lee, S.H.; Yoo, S.-M.; Cho, S.-M.; Cha, Y.-S.; Kang, M.-S. Anti-Obesity Effects of Petasites japonicus (Meowi) Ethanol Extract on RAW 264.7 Macrophages and 3T3-L1 Adipocytes and Its Characterization of Polyphenolic Compounds. Nutrients 2020, 12, 1261. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051261
Ahn EM, Asamenew G, Kim HW, Lee SH, Yoo S-M, Cho S-M, Cha Y-S, Kang M-S. Anti-Obesity Effects of Petasites japonicus (Meowi) Ethanol Extract on RAW 264.7 Macrophages and 3T3-L1 Adipocytes and Its Characterization of Polyphenolic Compounds. Nutrients. 2020; 12(5):1261. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051261
Chicago/Turabian StyleAhn, Eun Mi, Gelila Asamenew, Heon Woong Kim, Sang Hoon Lee, Seon-Mi Yoo, Soo-Muk Cho, Youn-Soo Cha, and Min-Sook Kang. 2020. "Anti-Obesity Effects of Petasites japonicus (Meowi) Ethanol Extract on RAW 264.7 Macrophages and 3T3-L1 Adipocytes and Its Characterization of Polyphenolic Compounds" Nutrients 12, no. 5: 1261. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051261





