The Impact of an Early Lifestyle Intervention on Pregnancy Outcomes in a Cohort of Insulin-Resistant Overweight and Obese Women
Abstract
:1. Introduction
2. Materials and Methods
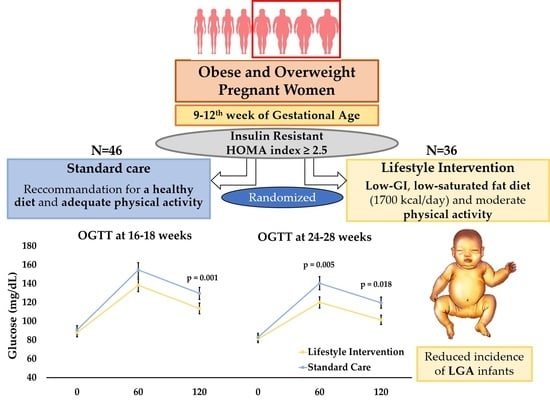
2.1. Study Design and Protocol
2.2. Study Procedures
2.2.1. Lifestyle Intervention
2.2.2. Standard Care
2.3. Outcomes Variables
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Maternal Outcomes
3.3. Oral Glucose-Tolerance Test
3.4. Neonatal Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Catalano, P. Obesity, Insulin Resistance and Pregnancy Outcome. Reproduction 2010, 140, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, P.; Ferrari, M.; Marconi, A. The relationship of maternal and fetal glucose concentrations in the human from midgestation until term. Metabolism 1988, 37, 358–363. [Google Scholar] [CrossRef]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (hapo) study: Associations with neonatal anthropometrics. Diabetes 2009, 58, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Chiefari, E.; Arcidiacono, B.; Foti, D. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef]
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes Mellitus and the Risk of Preterm Birth with Regard to the Risk of Spontaneous Preterm Birth. J. Matern. Fetal Neonatal Med. 2010, 23, 1004–1008. [Google Scholar] [CrossRef]
- Reece, E. The fetal and maternal consequences of gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2010, 23, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Bain, E.; Crane, M.; Tieu, J.; Han, S.; Crowther, C.; Middleton, P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2015, 12, CD010443. [Google Scholar] [CrossRef]
- Bruno, R.; Petrella, E.; Bertarini, V.; Pedrielli, G.; Neri, I.; Facchinetti, F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child Nutr. 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Vijai, V.; Petrella, E.; Zoccoli, S.G.; Pignatti, L.; Cerbo, L.D.; Neri, I. Food glycemic index changes in overweight/obese pregnant women enrolled in a lifestyle program: A randomized controlled trial. Am. J. Obstet. Gynecol. MFM 2019, 1, 100030. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care 2011, 34, S11–S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Clinical Health and Care Excellence. NICE guidance on diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. Lond. UK NICE Clin. Guidel. 2015, 15, NG3. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 190: Gestational diabetes mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- Bier, D.; Brosnan, J.; Flatt, J. Report of the IDECG Working Group on lower and upper limits of carbohydrate and fat intake. Eur. J. Clin. Nutr. 1999, 53, s177–s178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, K.; Takeda, K.; Maeda, M.; Ogawa, W.; Sato, T.; Okada, S.; Ohnishi, Y.; Nakajima, H.; Kashiwagi, A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol. Int. 2016, 7, 53–58. [Google Scholar] [CrossRef]
- Mammaro, A.; Carrara, S.; Cavaliere, A.; Pedata, R. Hypertensive Disorders of Pregnancy. J. Prenat. Med. 2009, 3, 1–5. [Google Scholar]
- Rasmussen, K.; Catalano, P.; Yaktinec, A. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr. Opin. Obstet. Gynecol. 2009, 21, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Johns, E.; Denison, F.; Norman, J.; Reynolds, R. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Denison, F.; Aedla, N.; Keag, O.; Hor, K.; Reynolds, R.; Milne, A.; Diamond, A.; Royal College of Obstetricians and Gynaecologists. Care of Women with Obesity in Pregnancy. Green-top Guideline No. 72. BJOG 2018. BJOG 2019, 126, e62–e106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Miller, A.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, R.; Luebcke, M.; Davis, W. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am. J. Clin. Nutr. 2006, 84, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.; Huston, L.; Amini, S.; Kalhan, S. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Yamashita, H.; Yasuhi, I.; Fukuda, M.; Kugishima, Y.; Yamauchi, Y.; Kuzume, A.; Hashimoto, T.; Sugimi, S.; Umezaki, Y.; Suga, S.; et al. The Association between Maternal Insulin Resistance in Mid-Pregnancy and Neonatal Birthweight in Uncomplicated Pregnancies. Endocr. J. 2014, 61, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, R.; Casey, S.; Quinn, E. Pregnancy and glycemic index outcomes study: Effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes. Am. J. Clin. Nutr. 2014, 99, 517–523. [Google Scholar] [CrossRef] [Green Version]
- McGowan, C.; McAuliffe, F. The influence of maternal glycaemia and dietary glycaemic index on pregnancy outcome in healthy mothers. Br. J. Nutr. 2010, 104, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Mitanchez, D.; Ciangura, C.; Jacqueminet, S. How Can Maternal Lifestyle Interventions Modify the Effects of Gestational Diabetes in the Neonate and the Offspring? A Systematic Review of Meta-Analyses. Nutrients 2020, 12, 353. [Google Scholar] [CrossRef] [Green Version]

| Standard Care (N = 46) | Lifestyle Intervention (N = 36) | p Value | |
|---|---|---|---|
| Mean Maternal Age (y) | 30.4 ± 5.5 | 30.5 ± 4.4 | 0.98 |
| Age Class | 0.97 | ||
| ≤25 | 8 (17.4%) | 5 (13.9%) | |
| 26–35 | 35 (76.1%) | 30 (83.4%) | |
| ≥36 | 3 (6.5%) | 1 (2.7%) | |
| Education | 0.34 | ||
| Low | 15 (32.6%) | 14 (38.9%) | |
| Medium | 26 (56.5%) | 15 (41.7%) | |
| High | 5 (10.9%) | 7 (19.4%) | |
| Occupation | 0.21 | ||
| Unemployed | 20 (43.5%) | 13 (36.1%) | |
| Housewife | 12 (26.1%) | 8 (22.2%) | |
| Employed | 14 (30.4%) | 15 (41.7%) | |
| Ethnicity | 0.19 | ||
| Caucasian | 32 (69.8%) | 29 (80.5%) | |
| African | 5 (10.8%) | 5 (13.9%) | |
| Sub-Saharan | 4 (8.6%) | 2 (5.6%) | |
| Other | 5 (10.8%) | 0 (0.0 %) | |
| Family history of hypertension | 18 (39.1%) | 17 (47.2%) | 0.46 |
| Family history of diabetes | 11 (30.5%) | 12 (33.3%) | 0.34 |
| Nulliparity | 18 (39.1%) | 18 (50.0%) | 0.32 |
| BMI | 0.69 | ||
| Overweight | 7 (15.2%) | 5 (13.9%) | |
| Obese | 39 (84.8%) | 31 (86.1%) | |
| Prepregnancy BMI (kg/m2) | 36.7 ± 5.9 | 37.4 ± 5.5 | 0.63 |
| Prepregnancy Weight (kg) | 99.5 ± 15.7 | 99.0 ± 17.4 | 0.90 |
| Standard Care (N = 46) | Lifestyle Intervention (N = 36) | p Value | |
|---|---|---|---|
| Gestational Weight Gain (kg) | 7.8 ± 7.2 | 6.7 ± 6.7 | 0.55 |
| Below Institute of Medicine (IOM) | 10 (21.8%) | 8 (22.2%) | 0.95 |
| Within IOM | 18 (39.1%) | 17 (47.2%) | 0.46 |
| Above IOM | 18 (39.1%) | 11 (30.5%) | 0.42 |
| Gestational Hypertensive Disorders | 4 (8.7%) | 6 (16.6%) | 0.27 |
| Gestational Diabetes Mellitus | 22 (47.8%) | 17 (47.2%) | 0.80 |
| Gestational Age at delivery (w) | 38.3 ± 2.01 | 38.4 ± 1.94 | 0.76 |
| Preterm Birth | 7 (15.5%) | 3 (8.3%) | 0.37 |
| Induction of Labor | 25 (54.3%) | 22 (61.1%) | 0.53 |
| Caesarean Section | 21 (46.7%) | 14 (38.9%) | 0.89 |
| Standard Care (N = 46) | Lifestyle Intervention (N = 36) | p Value | ||
|---|---|---|---|---|
| 16–18 weeks | Oral Glucose-Tolerance Test | |||
| 0 min (mg/dL) | 90.6 ± 14.7 | 87.5 ± 10.1 | 0.35 | |
| 60 min (mg/dL) | 154.5 ± 39.5 | 138.4 ± 34.9 | 0.22 | |
| 120 min (mg/dL) | 129.6 ± 42.1 | 113.3 ± 24.1 | 0.001 | |
| Area-under-curve glucose (mg min/dL) | 15,879.1 ± 3891.2 | 14,340.3 ± 2784.8 | 0.05 | |
| 24–28 weeks | Oral Glucose-Tolerance Test | |||
| 0 min (mg/dL) | 83.7 ± 7.3 | 81.8 ± 8.9 | 0.10 | |
| 60 min (mg/dL) | 141.8 ± 31.7 | 120.52 ± 47.8 | 0.005 | |
| 120 min (mg/dL) | 120.8 ± 28.1 | 101.38 ± 39.1 | 0.018 | |
| Area-under-curve glucose (mg min/dL) | 14,645.6 ± 2636.5 | 12,790.1 ± 2110.2 | 0.02 | |
| Standard Care (N = 46) | Lifestyle Intervention (N = 36) | p Value | |
|---|---|---|---|
| Birth Weight (g) | 3384.7 ± 648 | 3343.2 ± 669 | 0.77 |
| Macrosomia (>4000 g) | 7 (15.5%) | 3 (8.3%) | 0.34 |
| Large for Gestational Age | 12 (26.1%) | 3 (8.3%) | 0.04 |
| Small for Gestational Age | 6 (13.3%) | 2 (5.5%) | 0.25 |
| Apgar at 5 min <7 | 3 (6.6%) | 3 (8.3%) | 0.39 |
| pH < 7.1 | 2 (4.3%) | 2 (5.5%) | 0.80 |
| Resuscitation | 1 (2.2%) | 3 (8.3%) | 0.21 |
| Neonatal Intensive-Care-Unit Admission | 2 (4.4%) | 4 (11.1%) | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichini, D.; Petrella, E.; Dipace, V.; Di Monte, A.; Neri, I.; Facchinetti, F. The Impact of an Early Lifestyle Intervention on Pregnancy Outcomes in a Cohort of Insulin-Resistant Overweight and Obese Women. Nutrients 2020, 12, 1496. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051496
Menichini D, Petrella E, Dipace V, Di Monte A, Neri I, Facchinetti F. The Impact of an Early Lifestyle Intervention on Pregnancy Outcomes in a Cohort of Insulin-Resistant Overweight and Obese Women. Nutrients. 2020; 12(5):1496. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051496
Chicago/Turabian StyleMenichini, Daniela, Elisabetta Petrella, Vincenza Dipace, Alessia Di Monte, Isabella Neri, and Fabio Facchinetti. 2020. "The Impact of an Early Lifestyle Intervention on Pregnancy Outcomes in a Cohort of Insulin-Resistant Overweight and Obese Women" Nutrients 12, no. 5: 1496. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051496