Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
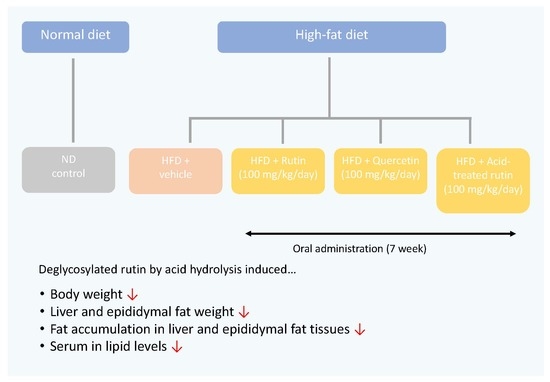
2.3. Animal Treatment
2.4. Sample Collection
2.5. Histopathology
2.6. Measurement of Plasma Biochemical Parameters
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Acid-Treated Rutin on Body Weight Gain and Food Intake
3.2. Effect of Acid-Treated Rutin on Histological Change in Epididymal Adipose Tissue and Liver
3.3. Effect of Acid-Treated Rutin on Serum Lipid Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Rigby, N.; Leach, R.; International Obesity Task Force. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J.; et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conforti, F.; Pan, M.H. Natural Products in Anti-Obesity Therapy. Molecules 2016, 21, 1750. [Google Scholar] [CrossRef]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural products in anti-obesity therapy. Nat. Prod. Rep. 2011, 28, 1493–1533. [Google Scholar] [CrossRef]
- La Casa, C.; Villegas, I.; Alarcon de la Lastra, C.; Motilva, V.; Martin Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- De Araujo, M.E.; Moreira Franco, Y.E.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Frankland Sawaya, A.C.; Ruiz, A.L.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Afanas’eva, I.B.; Ostrakhovitch, E.A.; Mikhal’chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef]
- Yuan, X.; Wei, G.; You, Y.; Huang, Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.; Zhang, C.; et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017, 31, 333–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of Rutin to Quercetin by Acid Treatment in Relation to Biological Activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Do, H.J.; Kim, O.Y.; Shin, M.J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. 2013, 58, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Gedikli, S.; Atila, G.; Uslu, H.; Makav, M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 2017, 7, 647–653. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Graham, T.E.; Kahn, B.B. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol. Metab. Syndr. 2012, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Britton, K.A.; Fox, C.S. Perivascular adipose tissue and vascular disease. Clin. Lipidol. 2011, 6, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Akil, L.; Ahmad, H.A. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J. Health Care Poor Underserved 2011, 22, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food. Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Osada, K.; Funayama, M.; Fuchi, S.; Sami, M.; Ohta, Y.; Kanda, T.; Ikeda, M. Effects of dietary procyanidins and tea polyphenols on adipose tissue mass and fatty acid metabolism in rats on a high fat diet. J. Oleo Sci. 2006, 55, 79–89. [Google Scholar] [CrossRef]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2016, 102, 188–202. [Google Scholar] [CrossRef]
- Madsen, A.N.; Hansen, G.; Paulsen, S.J.; Lykkegaard, K.; Tang-Christensen, M.; Hansen, H.S.; Levin, B.E.; Larsen, P.J.; Knudsen, L.B.; Fosgerau, K.; et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: A polygenetic rat model mimicking the human obesity syndrome. J. Endocrinol. 2010, 206, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.T.; Fong, W.P.; Cheung, Y.L.; Huang, Y.; Ho, W.K.; Chen, Z.Y. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J. Nutr. 1999, 129, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.H.; Lee, W.K.; Yi, B.R.; Lee, H.R.; Park, M.A.; Park, S.K.; Park, H.K.; Choi, K.C. Risk of cardiovascular disease is suppressed by dietary supplementation with protamine and chitooligosaccharide in Sprague-Dawley rats. Mol. Med. Rep. 2013, 7, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.I.; Lo, Y.C.; Su, N.W.; Chou, C.C.; Cheng, K.C. Enrichment of two isoflavone aglycones in black soymilk by immobilized beta-glucosidase on solid carriers. J. Agric. Food Chem. 2012, 60, 12540–12546. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, J.; Murphy, P.A.; Alekel, D.L.; Franke, W.D.; Hendrich, S. Rapid gut transit time and slow fecal isoflavone disappearance phenotype are associated with greater genistein bioavailability in women. J. Nutr. 2003, 133, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Kim, Y.J.; Lee, S.; Cho, E.J.; Park, S.H.; Ham, J.; Kim, H.Y.; Kang, K.S. Increase in antioxidant and anticancer effects of ginsenoside Re-lysine mixture by Maillard reaction. Food Chem. 2013, 138, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yamabe, N.; Choi, P.; Lee, J.W.; Ham, J.; Kang, K.S. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J. Agric. Food Chem. 2013, 61, 9185–9191. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef] [Green Version]



| Ingredient | Experimental Groups (1) | |
|---|---|---|
| ND | HFD | |
| Casein | 200 | 200 |
| L-Cysteine | 3 | 3 |
| Corn starch | 402.2 | 214.7 |
| Maltodextrin 10 | 70 | 100 |
| Sucrose | 172.8 | 172.8 |
| Cellulose | 50 | 50 |
| Soybean oil | 25 | 25 |
| Lard | 20 | 177.5 |
| Mineral Mix S10026 | 10 | 10 |
| Dicalcium phosphate | 13 | 13 |
| Calcium carbonate | 5.5 | 5.5 |
| Potassium citrate | 16.5 | 16.5 |
| Vitamin Mix V10001 | 10 | 10 |
| Choline Bitartrate | 2 | 2 |
| Groups | ND (g/mice) | HFD (g/mice) | R (g/mice) | AR(g/mice) | Q (g/mice) |
|---|---|---|---|---|---|
| 0 day | 22.95 ± 1.45 Ba | 23.60 ± 1.30 Ba | 22.72 ± 0.73 Ba | 22.80 ± 1.39 Ba | 23.13 ± 0.94 Ba |
| 3 days | 23.40 ± 1.55 Ba | 26.52 ± 1.48 Ba | 25.14 ± 1.26 Ba | 24.37 ± 1.68 Ba | 24.64 ± 1.49 Ba |
| 7 days | 24.14 ± 1.71 Ba | 27.43 ± 1.33 Ba | 26.53 ± 1.51 Ba | 25.41 ± 1.65 Ba | 25.66 ± 1.54 Ba |
| 14 days | 24.55 ± 1.74 Ba | 28.39 ± 1.27 Aa | 27.40 ± 1.37 Aa | 26.52 ± 2.12 Aa | 26.16 ± 1.86 Ba |
| 21 days | 24.90 ± 1.91 Bb | 30.10 ± 1.56 Aa | 28.77 ± 1.47 Aab | 27.40 ± 2.14 Aab | 27.66 ± 2.02 Aa |
| 28 days | 24.76 ± 2.16 Bb | 32.00 ± 2.19 Aa | 29.62 ± 1.97 Aa | 28.83 ± 2.75 Aa | 28.54 ± 1.98 Aab |
| 35 days | 26.28 ± 2.17 Bb | 33.70 ± 2.71 Aa | 30.99 ± 2.16 Aa | 29.67 ± 3.27 Aab | 29.83 ± 1.62 Aab |
| 42 days | 27.70 ± 1.79 Ab | 35.17 ± 2.70 Aa | 32.28 ± 2.94 Aa | 31.04 ± 3.43 Ab | 31.15 ± 2.02 Aa |
| 49 days | 27.33 ± 3.47 Ac | 38.12 ± 3.29 Aa | 34.07 ± 3.08 Ab | 32.73 ± 4.10 Ab | 33.01 ± 2.42 Ab |
| Group | Body Weight Gain (g/Mice/7 Weeks) | Food Intake (g/Mice/Day) | FER (%) (1) |
|---|---|---|---|
| ND | 4.37 ± 2.83 c | 3.09 ± 0.41 a | 2.51 ± 3.06 b |
| HFD | 14.52 ± 3.42 a | 2.70 ± 0.13 b | 8.96 ± 3.89 a |
| R | 11.36 ± 3.35 b | 2.55 ± 0.18 b | 7.23 ± 1.94 a |
| AR | 9.92 ± 4.48 b | 2.47 ± 0.24 b | 7.31 ± 2.32 a |
| Q | 9.87 ± 2.49 b | 2.52 ± 0.22 b | 7.04 ± 2.90 a |
| Group | Liver (g) | Epididymal Fat (g) |
|---|---|---|
| ND | 1.33 ± 0.15 b | 0.65 ± 0.24 c |
| HFD | 1.56 ± 0.15 a | 2.56 ± 0.40 a |
| R | 1.20 ± 0.13 bc | 2.08 ± 0.49 b |
| AR | 1.14 ± 0.17 c | 1.94 ± 0.44 b |
| Q | 1.21 ± 0.11 bc | 2.05 ± 0.42 b |
| Parameters | ND | HFD | R | AR | Q |
|---|---|---|---|---|---|
| AST (IU/L) | 94.14 ± 22.79 b | 133.81 ± 13.51 a | 94.59 ± 49.26 b | 126.26 ± 39.40 ab | 97.43 ± 47.31 b |
| ALT (IU/L) | 29.51 ± 4.58 c | 59.16 ± 20.44 a | 38.31 ± 10.17 c | 30.87 ± 9.78 bc | 41.53 ± 15.65 b |
| BUN (mg/dL) | 26.08 ± 4.75 a | 24.10 ± 5.73 a | 25.97 ± 5.39 a | 23.19 ± 3.72 a | 21.75 ± 3.72 a |
| CREA (mg/dL) | 0.44 ± 0.03 a | 0.43 ± 0.01 a | 0.41 ± 0.02 b | 0.40 ± 0.02 b | 0.40 ± 0.02 b |
| GLC (mg/dL) | 233.14 ± 31.63 b | 270.46 ± 38.89 ab | 274.04 ± 54.32 a | 272.72 ± 34.49 ab | 255.39 ± 37.89 ab |
| TG (mg/dL) | 45.49 ± 11.05 bc | 69.30 ± 20.33 a | 54.10 ± 13.05 ab | 36.82 ± 12.10 cd | 28.23 ± 11.26 d |
| TC (mg/dL) | 119.95 ± 11.86 c | 253.43 ± 24.68 a | 263.00 ± 22.13 a | 222.89 ± 26.42 b | 243.92 ± 23.85 a |
| LDL-c (mg/dL) | 10.90 ± 1.85 d | 27.26 ± 3.90 a | 26.26 ± 3.59 a | 18.58 ± 3.61 c | 22.60 ± 3.92 b |
| HDL-c (mg/dL) | 67.66 ± 6.56 c | 110.16 ± 9.86 ab | 111.86 ± 8.24 a | 102.63 ± 7.61 b | 106.23 ± 11.58 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Lee, J.; Kim, Y. Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 1539. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051539
Yang J, Lee J, Kim Y. Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice. Nutrients. 2020; 12(5):1539. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051539
Chicago/Turabian StyleYang, Jinwoo, Junsoo Lee, and Younghwa Kim. 2020. "Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice" Nutrients 12, no. 5: 1539. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051539