Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet
Abstract
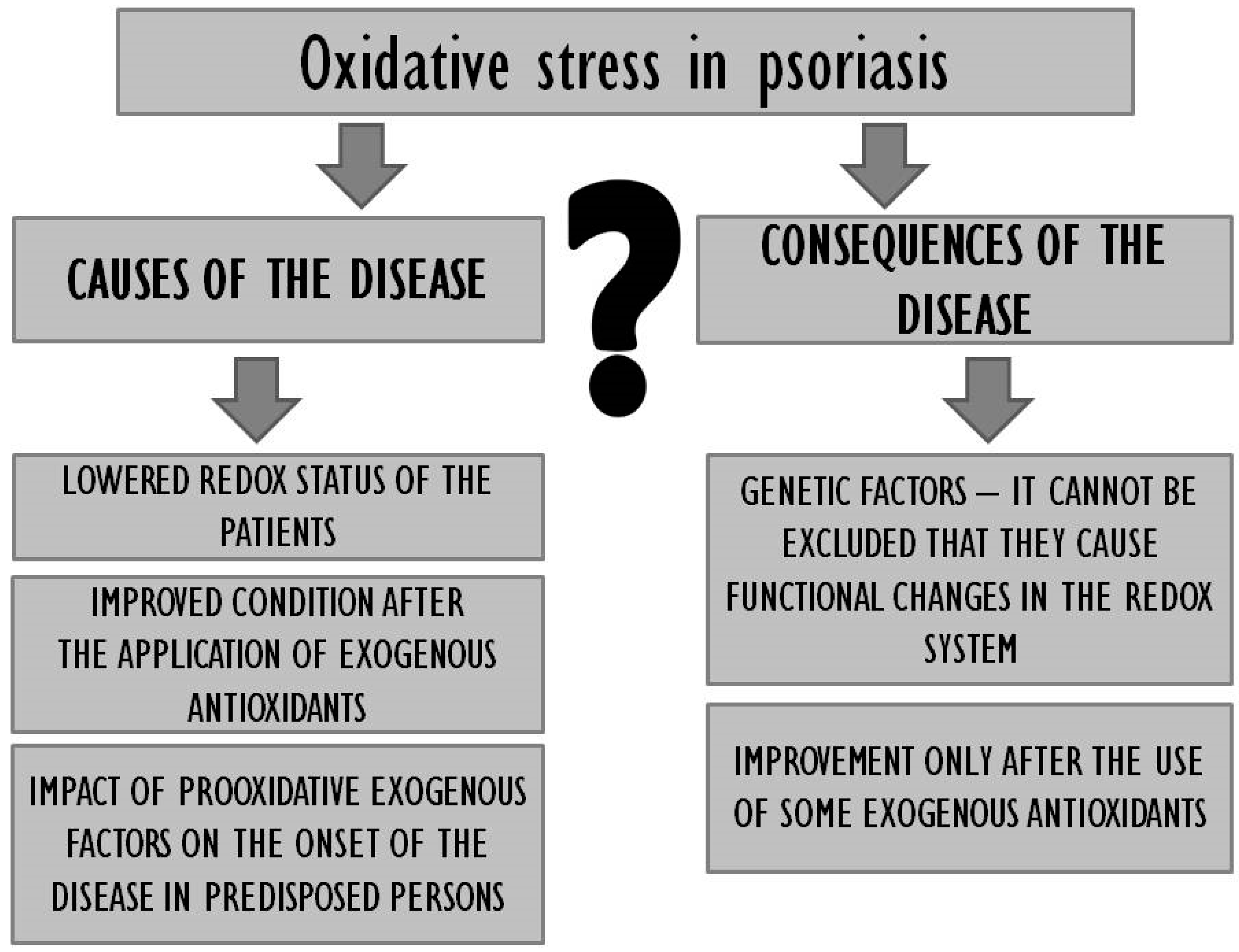
:1. Introduction
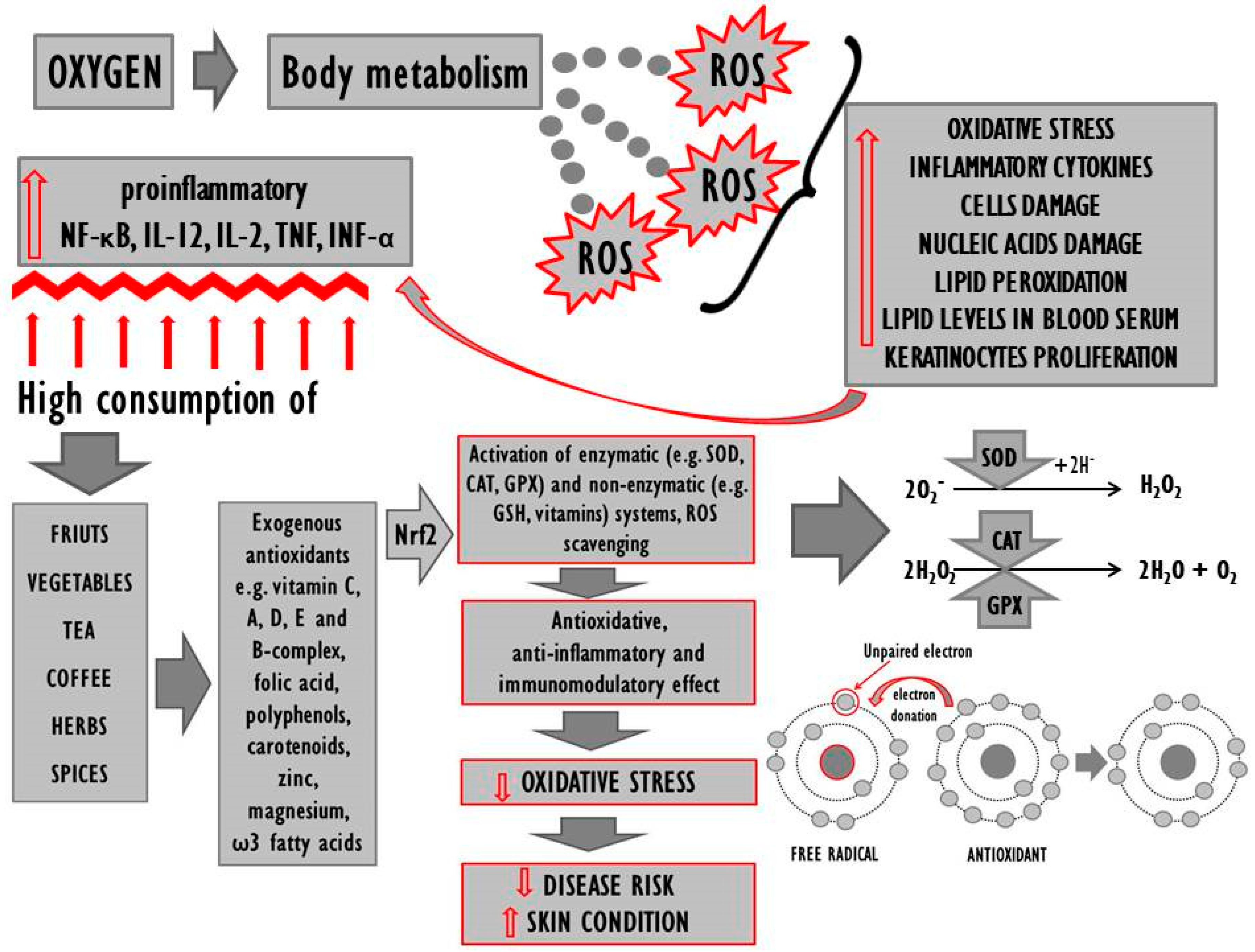
2. Systemic Oxidoreductive Balance
3. The Organism’s Antioxidative Status in Psoriasis
3.1. Blood and Serum
3.1.1. SOD, CAT and GPX Activity
3.1.2. Antioxidant Vitamins
3.1.3. Thiol/Disulphide Balance
3.1.4. Lipid Levels and Lipid Peroxidation Biomarkers
3.2. Epidermal Cells
3.3. Saliva
4. Exogenous Antioxidants in the Diet of Psoriasis Patients
4.1. Diet Structure
4.2. Food Containing Antioxidant Compounds
4.2.1. Tea
4.2.2. Coffee
4.2.3. Herbs and Spices
4.2.4. Fruit
5. External Application of Antioxidants in Psoriasis
6. Clinical Studies
7. Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Liao, W.; Chang, M.; Schrodi, S.J.; Bui, N.; Catanese, J.J.; Poon, A.; Matsunami, N.; Callis-Duffin, K.P.; Leppert, M.F.; et al. Further genetic evidence for three psoriasis-risk genes: ADAM33, CDKAL1, and PTPN22. J. Investig. Dermatol. 2009, 129, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Mrowietz, U.; Rostami-Yazdi, M. Oxidative stress in the pathogenesis of psoriasis. Free Radic. Biol. Med. 2009, 47, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Y.; Cheng, H.; Wang, W.J.; Wang, W.J.; Fu, H.Y.; Liu, L.H.; Zhang, F.Y.; Yang, S.; Zhang, X.J. TNIP1/ANXA6 and CSMD1 variants interacting with cigarette smoking, alcohol intake affect risk of psoriasis. J. Dermatol. Sci. 2013, 70, 94–98. [Google Scholar] [CrossRef]
- Capon, F. The genetic basis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Pedrajas, R.; Ramírez-Lamelas, D.T.; Muriach, B.; Sánchez-Villarejo, M.V.; Almansa, I.; Vidal-Gil, L.; Romero, F.J.; Barcia, J.M.; Muriach, M. Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front. Cell Neurosci. 2015, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [Green Version]
- Roszkiewicz, M.; Dopytalska, K.; Szymańska, E.; Jakimiuk, A.; Walecka, I. Environmental risk factors and epigenetic alternations in psoriasis. Ann. Agric. Environ. Med. 2019, 209184367, in press. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—a review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [Green Version]
- Partyka, A.; Czopek, A.; Stanisz-Wallis, K.; Zagórska, A. The use of biopharmaceuticals in the treatment of psoriasis. Postepy Hig. Med. Dosw. 2018, 72, 642–658. [Google Scholar] [CrossRef]
- Shilov, V.N.; Sergienko, V.I. Oxidative stress in keratinocytes as an etiopathogenetic factor of psoriasis. Bull. Exp. Biol. Med. 2000, 129, 364–369. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- de Gruijl, F.R.; van der Leun, J.C. Environment and health: 3. Ozone depletion and ultraviolet radiation. Can. Med. Assoc. J. 2000, 163, 851–855. [Google Scholar]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the skin: Understanding formulation and efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattahi, S.; Kazemipour, N.; Hashemi, M.; Sepehrimanesh, M. Alpha-1 antitrypsin, retinol binding protein and keratin 10 alterations in patients with psoriasis vulgaris, a proteomic approach. Iran. J. Basic Med. Sci. 2014, 17, 651–655. [Google Scholar]
- Rocha-Pereira, P.; Santos-Silva, A.; Rebelo, I.; Figueiredo, A.; Quintanilha, A.; Teixeira, F.J. The inflammatory response in mild and in severe psoriasis. Brit. J. Dermatol. 2004, 150, 917–928. [Google Scholar] [CrossRef]
- Ishibashi, T.; Ichikawa, M.; Sato, B.; Shibata, S.; Hara, Y.; Naritomi, Y.; Okazaki, K.; Nakashima, Y.; Iwamoto, Y.; Koyanagi, S.; et al. Improvement of psoriasis-associated arthritis and skin lesions by treatment with molecular hydrogen: A report of three cases. Mol. Med. Rep. 2015, 12, 2757–2764. [Google Scholar] [CrossRef] [Green Version]
- Mailloux, R.J.; Xiaolei, J.; Willmore, W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014, 2, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Mikhed, Y.; Daiber, A.; Steven, S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015, 16, 15918–15953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The role of nutrients in protecting mitochondrial function and neurotransmitter signaling: Implications for the treatment of depression, ptsd, and suicidal behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizilyel, O.; Akdeniz, N.; Metin, M.S.; Elmas, Ö.F. Investigation of oxidant and antioxidant levels in patients with psoriasis. Turk. J. Med. Sci. 2019, 49, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, M.; Mila-Kierzenkowska, C.; Kwiatkowska, A.; Paprocki, J.; Sutkowy, P.; Woźniak, A. Evaluation of selected parameters of oxidative stress in patients with psoriasis. Diagn. Lab. 2016, 52, 101–106. [Google Scholar]
- Priya, R.; Kumar, U.; Saran, A.; Kumari, R.; Koschore, C. Oxidative stress in psoriasis. Biomed. Res. 2013, 25, 132–134. [Google Scholar]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflam. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yan, T.; Yang, J.Q.; Oberley, T.D.; Oberley, L.W. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000, 60, 3927–3939. [Google Scholar]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Pouya, V.T.; Hashemy, I.; Shoeibi, A.; Tirkani, A.N.; Tavallaie, S.; Avval, F.Z.; Soukhtanloo, M.; Mashkani, B.; Alamdari, D.H. Serum pro-oxidant-antioxidant balance, advanced oxidized protein products (AOPP) and protein carbonyl in patients with stroke. Razavi Int. J. Med. 2016, 4, e38203. [Google Scholar]
- Bradshaw, P.C. Cytoplasmic and mitochondrial NADPH-Coupled redox systems in the regulation of aging. Nutrients 2019, 11, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondei, L.S.; Silveira, L.M.; Leite, A.A.; Souza, D.R.S.; Pinhel, M.A.S.; Percário, S.; Ricci Júnior, O.; Bonini-Domingos, C.R. Lipid peroxidation and antioxidant capacity of G6PD-deficient patients with A-(202G>A) mutation. Genet. Mol. Res. 2009, 8, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of oxidative stress in various stages of psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef] [Green Version]
- Kute, P.K.; Muddeshwar, M.G.; Sonare, A.R. Pro-oxidant and anti-oxidant status in patients of psoriasis with relation to smoking and alcoholism. J. Evol. Med. Dent. Sci. 2019, 8, 2677–2680. [Google Scholar]
- Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered lipid metabolism in blood mononuclear cells of psoriatic patients indicates differential changes in psoriasis vulgaris and psoriatic arthritis. Int. J. Mol. Sci. 2019, 20, 4249. [Google Scholar] [CrossRef] [Green Version]
- Pujari, V.M.; Ireddy, S.; Itagi, I.; Siddesh, K.H. The serum levels of malondialdehyde, vitamin E and erythrocyte catalase activity in psoriasis patients. J. Clin. Diagn. Res. 2014, 8, 14–16. [Google Scholar] [CrossRef]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary antioxidants and oxidative stress in psoriatic patients: Can salivary total oxidant status and oxidative status index be a plaque psoriasis biomarker? Oxid. Med. Cell. Longev. 2020, 2020, 9086024. [Google Scholar] [CrossRef] [Green Version]
- Alwasiti, E.A.R.K.; Al-Rubayee, W.T.; Al-Tammimy, S.M. Serum copper, zinc and oxidative stress in patients with psoriasis. Iraqi. J. Med. Sci. 2011, 9, 137–142. [Google Scholar]
- Üstüner, P.; Balevi, A.; Özdemir, M.; Olmusçelik, O.; Ülfer, G.; Yigitbasi, T. The role of thiol/disulfide homeostasis in psoriasis: Can it be a new marker for inflammation? Turk. Arch. Dermatol. Venerol. Turkderm 2018, 52, 120–125. [Google Scholar] [CrossRef]
- Aksoy, M.E.; Kirmit, A. Thiol/disulphide balance in patients with psoriasis. Adv. Dermatol. Allergol. 2018, 37, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, A.; Yorulmaz, A.; Erdogan, S.; Cakmak, S.K.; Guney, E.; Sen, O.; Erel, O. An evaluation of thiol/disulphide homeostasis in patients with psoriasis. Adv. Dermatol. Allergol. 2017, 5, 464–467. [Google Scholar] [CrossRef] [Green Version]
- Boda, D.; Negrei, C.; Nicolescu, F.; Bălălău, C. Assessment of some oxidative stress parameters in methotrexate treated psoriasis patients. Farmacia 2014, 62, 704–710. [Google Scholar]
- Nemati, H.; Khodarahmi, R.; Sadeghi, M.; Ebrahimi, A.; Rezaei, M.; Vaisi-Raygani, A. Antioxidant status in patients with psoriasis. Cell Biochem. Funct. 2014, 32, 268–273. [Google Scholar]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Kiciński, P.; Pietrzak-Franciszkiewicz, K.; Krasowska, D.; Kandzierski, G. Serum lipid metabolism in psoriasis and psoriatic arthritis—an update. Arch. Med. Sci. 2019, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 677–679. [Google Scholar]
- Kiran, P.U.; Deedi, M.K. Study of MDA and SGPT levels in patients with psoriasis. J. Evol. Res. Med. Biochem. 2015, 1, 9–13. [Google Scholar]
- Abdel-Mawla, M.Y.; Nofal, E.; Khalifa, N.; Abdel-Shakoor, R.; Nasr, M. Role of oxidative stress in psoriasis: An evaluation study. J. Am. Sci. 2013, 9, 151–155. [Google Scholar]
- Metta, S.; Kumar, M.A.; Basalingappa, R.D.; Uppala, S.; Mohanty, S. Circulatory markers of oxidative stress and dyslipidemia in male patients of chronic plaque psoriasis. Int. J. Med. Public Health 2015, 5, 208–212. [Google Scholar]
- Kaur, M.; Sharma, S.; Kukreja, S.; Kaur, J.; Bassi, R. Study of oxidative stress in patients of psoriasis. Int. J. Res. Dermatol. 2016, 2, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Altu’ma, F.J.; Al-Hassan, A.T.A.; Al-Da’amy, A.M.A. Correlation between malondialdehyde and dyslipidemia in psoriatic patients. J. Contemp. Med. Sci. 2016, 2, 56–58. [Google Scholar]
- Şikar Aktürk, A.; Özdoğan, H.K.; Bayramgürler, D.; Çekmen, M.B.; Bilen, N.; Kıran, R. Nitric oxide and malondialdehyde levels in plasma and tissue of psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Chari, S.; Borkar, M.; Chandankhede, M. Dyslipidemia and oxidative stress in patients of psoriasis. Biomed. Res. 2011, 22, 222–225. [Google Scholar]
- Johnson, J.A.; Ma, C.; Kanada, K.N.; Armstrong, A.W. Diet and nutrition in psoriasis: Analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bojarowicz, H.; Woźniak, B. Polyunsaturated fatty acids and their influence on skin condition. Probl. Hig. Epidemiol. 2008, 89, 471–475. [Google Scholar]
- Kadam, D.; Lele, S.S. Extraction, characterization and bioactive properties of Nigella sativa seedcake. J. Food Sci. Technol. 2017, 54, 3936–3947. [Google Scholar] [CrossRef]
- Kokcam, I.; Naziroglu, M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin. Chim. Acta 1999, 289, 23–31. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campanati, A.; Ferretti, G.; Simonetti, O.; Liberati, G.; Offidani, A.M. Oxidative stress and psoriasis: The effect of antitumour necrosis factor-α inhibitor treatment. Br. J. Dermatol. 2013, 168, 984–989. [Google Scholar] [CrossRef]
- Barygina, V.; Becatti, M.; Lotti, T.; Taddei, N.; Fiorillo, C. Low dose cytokines reduce oxidative stress in primary lesional fibroblasts obtained from psoriatic patients. J. Dermatol. Sci. 2016, 83, 242–244. [Google Scholar] [CrossRef]
- Tekin, N.S.; Tekin, I.O.; Barut, F.; Sipahi, E.Y. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediat. Inflamm. 2007, 2007, 78454. [Google Scholar]
- Portugal-Cohen, M.; Horev, L.; Ruffer, C.; Schlippe, G.; Voss, W.; Ma’or, Z.; Oron, M.; Soroka, Y.; Frušić-Zlotkin, M.; Milner, Y.; et al. Non-invasive skin biomarkers quantification of psoriasis and atopic dermatitis: Cytokines, antioxidants and psoriatic skin auto-fluorescence. Biomed. Pharmacother. 2012, 66, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, B.; Lee, Y.S.; Kim, T.Y. Role of superoxide dismutase 3 in skin inflammation. J. Dermatol. Sci. 2012, 67, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.S.; Adil, M.; Alam, M. Role of dietary intervention in psoriasis: A review. Ind. J. Clin. Dermatol. 2017, 1, 1–5. [Google Scholar]
- Duarte, G.; Barbosa, L.O.; Rosa, M.E.A. The management of psoriasis through diet. Psoriasis 2012, 2, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.P.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Efficacy of nutritional treatment in patients with psoriasis: A case report. Exp. Therap. Med. 2015, 10, 1071–1073. [Google Scholar] [CrossRef]
- Kim, M.K.; Cho, S.W.; Park, Y.K. Long-term vegetarians have low oxidative stress, body fat, and cholesterol levels. Nutr. Res. Pract. 2012, 6, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Peluso, I.; Raguzzini, A.; Catasta, G.; Cammisotto, V.; Perrone, A.; Tomino, C.; Toti, E.; Serafini, M. Effects of high consumption of vegetables on clinical, immunological, and antioxidant markers in subjects at risk of cardiovascular diseases. Oxid. Med. Cell. Longev. 2018, 2018, 5417165. [Google Scholar] [CrossRef] [PubMed]
- Nälsén, C.; Basu, S.; Wolk, A.; Vessby, B. The importance of dietary antioxidants on plasma antioxidant capacity and lipid peroxidation in vivo in middle-aged men. Scand. J. Food Nutr. 2006, 50, 64–70. [Google Scholar] [CrossRef]
- Araujo, M.L.; Burgos, M.G.; Moura, I.S. Nutritional influences in psoriasis. An. Bras. Dermatol. 2009, 84, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Mari, N.L.; Santos, L.F.R.F.; Alfieri, D.F.; Fauzino, T.; Lozovoy, M.A.B.; Martin, L.M.M.; Kipper, J.P.; Lena, C.P.; da Silva Taguti, P.; Colado Simão, A.N.; et al. Supplementation with fish oil improves life quality, and decreases inflammatory status and oxidative stress in psoriasis. Clin. Res. Trials 2018, 4, 1–7. [Google Scholar]
- Solis, M.Y.; de Melo, N.S.; Macedo, M.E.; Carneiro, F.P.; Sabbag, C.Y.; Lancha Júnior, A.H.; Frangella, V.S. Nutritional status and food intake of patients with systemic psoriasis and psoriatic arthritis associated. Einstein 2012, 10, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.; Napolitano, M.; Savanelli, M.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Translat. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, B.F.; Morimoto, M.A.; Gomes, C.; de Carvalho Klemz, B.N.; de Souza Genaro, P.; Teixeira Damasceno, N.R.; Szejnfeld, V.L.; de Medeiros Pinheiro, M. Higher bodily adiposity, fat intake, and cholesterol serum levels are associated with higher disease activity in psoriatic arthritis patients: Is there a link among fat and skin and joint involvement? Lipids Health Dis. 2020, 19, 21. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Morita, T.; Ito, M.; Okazaki, S.; Koto, M.; Ichikawa, Y.; Takayama, R.; Hoashi, T.; Saeki, H.; Kanda, N. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: Low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J. Dermatol. 2019, 46, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Afifi, L.; Danesh, M.J.; Lee, K.M.; Beroukhim, K.; Farahnik, B.; Ahn, R.S.; Yan, D.; Singh, R.K.; Nakamura, M.; Koo, J.; et al. Dietary behaviors in psoriasis: Patient-reported outcomes from a U.S. national survey. Dermatol. Ther. 2017, 7, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Al-Katib, S.R.; Al-Wakeel, H.A.; Al-Rawaf, R.F. Role of vitamin C as antioxidant in psoriasis patients treated with NB-UVB phototherapy. Indian J. Public Health Res. Dev. 2018, 9, 375–380. [Google Scholar] [CrossRef]
- Di Nardo, V.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lott, T. Use of curcumin in psoriasis. Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell. Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral curcumin (Meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. BioMed Res. Int. 2015, 2015, 283634. [Google Scholar] [CrossRef]
- Moghadam, A.R.; Tutunchi, S.; Namvaran-Abbas-Abad, A.; Yazdi, M.; Bonyadi, F.; Mohajeri, D.; Mazani, M.; Marzban, H.; Łos, M.J.; Ghavami, S.; et al. Pre-administration of turmeric prevents methotrexate-induced liver toxicity and oxidative stress. BMC Complement. Altern. Med. 2015, 15, 246. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, F.; Lavi Arab, F.; Jaafari, M.R.; Rastin, M.; Tabasi, N.; Hatamipour, M.; Nikkhah, K.; Mahmoudi, M. Immunoregulatory, proliferative and anti-oxidant effects of nanocurcuminoids on adipose-derived mesenchymal stem cells. EXCLI J. 2019, 18, 405–421. [Google Scholar] [PubMed]
- Chen, H.; Liu, H.; Lu, C.; Wang, M.; Li, X.; Zhao, H.; Yan, Y.; Yu, W.; Han, L.; Dai, Z. PSORI-CM02 formula increases CD4+ Foxp3+ regulatory T cell frequency and ameliorates imiquimod-induced psoriasis in mice. Front. Immunol. 2018, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Al-Jebori, A.A. Serum selenium in psoriatic patients. Iraqi J. Comm. Med. 2007, 20, 409–413. [Google Scholar]
- Nazıroğlu, M.; Yıldız, K.; Tamtürk, B.; Erturan, İ.; Flores-Arce, M. Selenium and psoriasis. Biol. Trace Elem. Res. 2012, 150, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Serwin, A.B.; Wasowicz, W.; Chodynicka, B. Selenium supplementation, soluble tumor necrosis factor-alpha receptor type 1, and C-reactive protein during psoriasis therapy with narrowband ultraviolet B. Nutrition 2006, 22, 860–864. [Google Scholar] [CrossRef]
- Nga, N.T.T.; An, V.T.; Quang, D.D. Antioxidant properties of folic acid: A DFT study. Viet. J. Sci. Technol. 2018, 56, 39–45. [Google Scholar]
- Aronson, P.J. Cases of psoriasis improved by lowering homocysteine using 4–7 mg folic acid, vitamins B6 and B12 previously worsened using 1–2 mg daily folic acid, B6 and B12 folic acid. J. Transl. Sci. 2017, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hicks, A. Current status and future development of global tea production and tea products. AU JT 2009, 12, 251–264. [Google Scholar]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Bhattacharjee, C. Extraction of polyphenols from dried tea leaves. J. Sci. Eng. Res. 2012, 3, 1–5. [Google Scholar]
- Serafini, M.; Ghiselli, A.; Ferro Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar] [PubMed]
- Gawlik, M.; Czajka, A. The effect of green, black and white tea on the level of α and γ tocopherols in free radical-induced oxidative damage of human red blood cells. Acta Pol. Pharm. 2007, 64, 159–164. [Google Scholar] [PubMed]
- Sung, H.; Nah, J.; Chun, S.; Park, H.; Yang, S.E.; Min, W.K. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000, 54, 527–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ. Toxicol. Pharmacol. 2013, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Wang, R.; Sun, P.; Zhao, X. Antioxidant-mediated preventative effect of Dragon-pearl tea crude polyphenol extract on reserpine-induced gastric ulcers. Exp. Ther. Med. 2015, 10, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef] [Green Version]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Nebesny, E.; Budryn, G. Antioxidative activity of green and roasted coffee beans as influenced by convection and microwave roasting methods and content of certain compounds. Eur. Food Res. Technol. 2003, 217, 157–163. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. Int. J. Food Prop. 2014, 17, 86–92. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teekachunhatean, S.; Tosri, N.; Sangdee, C.; Wongpoomchai, R.; Ruangyuttikarn, W.; Puaninta, C.; Srichairatanakool, S. Antioxidant effects after coffee enema or oral coffee consumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012, 31, 643–651. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular antioxidant and anti-inflammatory effects of coffee extracts with different roasting levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Danila, A.O.; Gatea, F.; Radu, G.L. Polyphenol composition and antioxidant activity of selected medicinal herbs. Chem. Nat. Comp. 2011, 47, 22–26. [Google Scholar] [CrossRef]
- Iskender, H.; Yenice, G.; Dokumacioglu, E.; Kaynar, O.; Hayirli, A.; Kaya, A. The effects of dietary flavonoid supplementation on the antioxidant status of laying hens. Braz. J. Poult. Sci. 2016, 18, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.K.; Raina, R.; Sultana, M.; Singh, M.; Kumar, P. Total antioxidant and oxidant status of plasma and renal tissue of cisplatin-induced nephrotoxic rats: Protection by floral extracts of Calendula officinalis Linn. Ren. Fail. 2016, 38, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed. Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef] [Green Version]
- Gümüş, R.; Erol, S.H.; İmik, H.; Halici, M. The effects of the supplementation of lamb rations with oregano essential oil on the performance, some blood parameters and antioxidant metabolism in meat and liver tissues. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 395–401. [Google Scholar]
- Gümüş, R.; Ercan, N.; Imik, H. The effect of thyme essential oil (Thymus vulgaris) added to quail diets on performance, some blood parameters, and the antioxidative metabolism of the serum and liver tissues. Braz. J. Poult. Sci. 2017, 19, 297–304. [Google Scholar] [CrossRef] [Green Version]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaney, D.M.; El-Far, A.H.; Sadek, K.M.; El-Sayed, Y.S.; Abdel-Latif, M.A. Impact of dietary thyme (Thymus vulgaris) on broiler chickens concerning immunity, antioxidant status, and performance. Alex. J. Vet. Sci. 2017, 55, 169–179. [Google Scholar]
- Hoseini, S.M.; Yousefi, M. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 2019, 25, 298–309. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC-MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug. Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Rašković, A.; Pavlović, N.; Kvrgić, M.; Sudji, J.; Mitić, G.; Čapo, I.; Mikov, M. Effects of pharmaceutical formulations containing thyme on carbon tetrachloride-induced liver injury in rats. BMC Complement. Altern. Med. 2015, 15, 442. [Google Scholar] [CrossRef] [Green Version]
- Palabiyik, S.S.; Karakus, E.; Halici, Z.; Cadirci, E.; Bayir, Y.; Ayaz, G.; Cinar, I. The protective effects of carvacrol and thymol against paracetamol-induced toxicity on human hepatocellular carcinoma cell lines (HepG2). Hum. Exp. Toxicol. 2016, 35, 1252–1263. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Abd-Algader, N.N.; El-kamali, H.H.; Ghanem, K.Z.; Farrag, A.R.H. Volatile compounds and antioxidant activity of the aromatic herb Anethum graveolens. J. Arab Soc. Med. Res. 2013, 8, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2019, 30912576, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moselhy, S.S.; Ali, H.K.H. Hepatoprotective effect of Cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol. Res. 2009, 42, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Khaki, A. Effect of Cinnamomum zeylanicumon on spermatogenesis. Iran Red. Crescent Med. J. 2015, 17, e18668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxidative Med. Cell. Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef] [PubMed]
- Roussel, A.M.; Hininger, I.; Benaraba, R.; Ziegenfuss, T.N.; Anderson, R.A. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J. Am. Coll. Nutr. 2009, 28, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ervina, M.; Nawu, Y.E.; Esar, S.Y. Comparison of in vitro antioxidant activity of infusion, extract and fractions of Indonesian cinnamon (Cinnamomum burmannii) bark. Int. Food Res. J. 2016, 23, 1346–1350. [Google Scholar]
- Settharaksa, S.; Jongjareonrak, A.; Hmadhlu, P.; Chansuwan, W.; Siripongvutikorn, S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012, 19, 1581–1587. [Google Scholar]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Topal, M.; Bingol, Z.; Cetin Çakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22, 1511–1526. [Google Scholar] [CrossRef] [Green Version]
- Naji, K.M.; Al-Shaibani, E.S.; Alhadi, F.A.; Al-Soudi, S.A.; D’souza, M.R. Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement. Altern. Med. 2017, 17, 411. [Google Scholar]
- Zamani, A.; Ghiasvand, T.; Hadei, J.; Babaahmadi-Rezaei, H.; Pishdadian, A. Effect of garlic consumption on total antioxidant status and some biochemical and haematological parameters in blood of rats. J. Sci. Food Agric. 2009, 89, 1434–1437. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asdaq, S.M.B. Antioxidant and hypolipidemic potential of aged garlic extract and its constituent, S-allyl cysteine, in rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 328545. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Dai, C.H.; Hou, F.R.; Zhu, P.P.; He, R.H.; Ma, H.L. A study on the ageing method and anti-oxidant activity of black garlic residues. Czech J. Food Sci. 2018, 36, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kim, M.R.; Cho, S.M.; Kim, S.Y.; Kim, J.B.; Cho, Y.S. Antioxidant activities and determination of phenolic compounds isolated from oriental plums (Soldam, Oishiwase and Formosa). Nutr. Res. Pract. 2012, 6, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Lv, R.; Wang, L.; Hou, H.; Liu, H.; Shao, S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018, 25, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Marniemi, J.; Hakala, P.; Maki, J.; Ahotupa, M. Partial resistance of low density lipoprotein to oxidation in vivo after increased intake of berries. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 331–337. [Google Scholar]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar]
- El-Wakf, A.M.; El-Komy, M.A.; Hassan, D.G. Preventive effect of dried plum extract against dexamethasone-induced osteoporosis in male rats through inhibiting cathepsin-K activity, lipogenesis and trabecular bone loss. J. Innov. Pharmace. Biol. Sci. 2019, 6, 52–61. [Google Scholar]
- Graziani, G.; D’Argenio, G.; Tuccillo, C.; Loguercio, C.; Ritieni, A.; Morisco, F.; Del Vecchio Blanco, C.; Fogliano, V.; Romano, M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 2005, 54, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of pomegranate juice supplementation on oxidative stress biomarkers following weightlifting exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doostan, F.; Vafafar, R.; Zakeri-Milani, P.; Pouri, A.; Amini Afshar, R.; Mesgari Abbasi, M. Effects of pomegranate (Punica granatum L.) seed and peel methanolic extracts on oxidative stress and lipid profile changes induced by methotrexate in rats. Adv. Pharm. Bull. 2017, 7, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doostan, F.; Abbasi, M.M.; Khordadmehr, M.; Fallah, F.; Behrouzy, A. Effects of pomegranate seed and peel methanolic extracts on methotrexate-induced hepatotoxicity in rats. Pharm. Sci. 2019, 25, 111–117. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC-MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Anahita, A.; Asmah, R.; Fauziah, O. Evaluation of total phenolic content, total antioxidant activity, andantioxidant vitamin composition of pomegranate seed and juice. Gen. Med. 2015, 3, 1000164. [Google Scholar]
- Weng, Z.; Patel, A.B.; Vasiadi, M.; Therianou, A.; Theoharides, T.C. Luteolin inhibits human keratinocyte activation and decreases NF-κB induction that is increased in psoriatic skin. PLoS ONE 2014, 9, e90739. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, W.; Yuhai; Wang, H.; Bagenna, C. Astilbin reduces ROS accumulation and VEGF expression through Nrf2 in psoriasis-like skin disease. Biol. Res. 2019, 52, 49. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, A.; Grenier, A.; Simard, F.; Gendreau, I.; Pichette, A.; Legault, J.; Pouliot, R. Dihydrochalcone derivatives from Populus balsamifera L. buds for the treatment of psoriasis. Int. J. Mol. Sci. 2019, 21, 256. [Google Scholar] [CrossRef] [Green Version]
- Skurić, J.; Orsolić, N.; Kolarić, D.; Dikić, D.; Benković, V.; Knezević, A.H.; Lisicić, D. Effectivity of flavonoids on animal model psoriasis—thermographic evaluation. Period Biol. 2011, 113, 457–463. [Google Scholar]
- Kjær, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar]
- Alashqar, M.B.; Goldstein, N. Caffeine in the treatment of atopic dermatitis and psoriasis: A review. SKIN J. Cutan. Med. 2019, 3, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.T.; Fox, R.J. BG-12 in multiple sclerosis. Semin. Neurol. 2013, 33, 56–65. [Google Scholar] [PubMed] [Green Version]
- Brigelius-Flohé, R.; Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef] [Green Version]
- Madhulatha, M.; Vijayabhaskar, M. Evaluation of therapeutic efficacy of antioxidants in psoriatic cases. Indian J. Med. Biochem. 2019, 23, 167–169. [Google Scholar] [CrossRef]
- Kharaeva, Z.; Gostova, E.; De Luca, C.; Raskovic, D.; Korkina, L. Clinical and biochemical effects of coenzyme Q(10), vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009, 25, 295–302. [Google Scholar] [CrossRef]
- Jin, G.H.; Liu, Y.; Jin, S.Z.; Liu, X.D.; Liu, S.Z. UVB induced oxidative stress in human keratinocytes and protective effect of antioxidant agents. Radiat. Environ. Biophys. 2007, 46, 61–68. [Google Scholar] [CrossRef]
- Mitra, A.; Fallen, R.S.; Lima, H. Cytokine-based therapy in Psoriasis. Clin. Rev. Allergy Immunol. 2012, 44, 173–182. [Google Scholar] [CrossRef]
- Barygina, V.; Becatti, M.; Soldi, G.; Prignano, F.; Lotti, T.; Nassi, P.; Wright, D.; Taddei, N.; Fiorillo, C. Altered redox status in the blood of psoriatic patients: Involvement of NADPH oxidase and role of anti-TNF-a therapy. Redox Rep. 2013, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chatterjee, M.P. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Azhar, I.Y.; Khalil, A.H. Evaluation of efficacy, safety and antioxidant effect of Nigella sativa in patients with psoriasis: A randomized clinical trial. J. Clin. Exp. Investig. 2014, 5, 186–193. [Google Scholar]
- Abbas, Y.M.; Ahmed, J.H.; Al-Haroon, S.S. A study of the effect of Nigella sativa (black seeds) on methotrexate induced hepatotoxicity in rabbits. Int. J. Basic Clin. Pharmacol. 2015, 4, 230–235. [Google Scholar] [CrossRef]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Sicak, Y.; Erdoğan Eliuz, E.A. Chemical content and biological activity spectrum of Nigella sativa seed oil. KSU J. Agric. Nat. 2019, 22, 928–934. [Google Scholar]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar]
- Polovynkina, K.; Klonowska, J.; Grzyb, S. General characteristics of therapeutic molecular hydrogen. Eur. J. Med. Technol. 2019, 3, 38–43. [Google Scholar]
- Zhu, Q.; Wu, Y.; Li, Y.; Chen, Z.; Wang, L.; Xiong, H.; Dai, E.; Wu, J.; Fan, B.; Ping, L.; et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci. Rep. 2018, 8, 8051. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaros, J.; Katta, R.; Shi, V.Y. Dermatonutrigenomics: Past, present, and future. Dermatology 2019, 235, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Switzeny, O.J.; Müllner, E.; Wagner, K.; Brath, H.; Aumüller, E.; Haslberger, A.G. Vitamin and antioxidant rich diet increases MLH1 promoter DNA methylation in DMT2 subjects. Clin. Epigenet. 2012, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, J.E.; Nguyen, G.H.; Fujita, M.; Florell, S.R.; Callis Duffin, K.; Krueger, G.G.; O’Connell, R.M. MicroRNAs in psoriasis. J. Invest Dermatol. 2016, 136, 365–371. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Reis, B.Z.; Duarte, G.B.S.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef]
- Bhardwaj, N. MicroRNAs in atopic dermatitis: A review. J. Transl. Genet. Genom. 2017, 1, 15–22. [Google Scholar]
- Kocic, H.; Damiani, G.; Stamenkovic, B.; Tirant, M.; Jovic, A.; Tiodorovic, D.; Peris, K. Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319864805. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Liu, X.; Wang, X.; Lv, H.; Zhao, J.; Guo, X.; Xian, F.; Ji, Y.; Zhan, G. Plasma microRNA expression profiles in psoriasis. J. Immunol. Res. 2020, 2020, 1561278. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.K.; Shao, S.; Zhao, Y.; Marincola, F.; Pesatori, A.; Bertazzi, P.A.; Caporaso, N.E.; Wang, E.; Landi, M.T. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2176–2184. [Google Scholar] [CrossRef] [Green Version]
- Lovendorf, M.B.; Zibert, J.R.; Hagedorn, P.H.; Glue, C.; Ødum, N.; Røpke, M.A.; Skov, L. Comparison of microRNA expression using different preservation methods of matched psoriatic skin samples. Exp. Dermatol. 2012, 21, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Li, H.; Jia, Z.; Li, A.; Jenkins, G.; Yang, X.; Hu, J.; Guo, W. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol. Cell. Biochem. 2013, 382, 137–143. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tili, E.; Michaille, J.-J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Schumacker, P.T.; Chandel, N.S. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol. Cell. Biol. 2007, 27, 5737–5745. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Antioxidant Capacity | Lipid Levels | Peroxidation Biomarkers | References |
|---|---|---|---|---|
| Psoriasis n = 26 Control n = 16 | ≈ SOD | ↑ MDA | [25] | |
| Psoriasis n = 90 Control n = 30 | ↓ SOD; ↓ CAT; ↑ NO; ↓ TAS | ↑ MDA | [34] | |
| Psoriasis n = 90 Control n = 90 | ↓ vitamin E; ↓ CAT | ↑ MDA | [37] | |
| Psoriasis n = 50 Control n = 50 | ↓ SOD; ↓ GPX | ↑ MDA | [35] | |
| Psoriasis n = 40 Control n = 40 | ↑ TG; ↑ TC | ↑ MDA | [38] | |
| Psoriasis n = 48 Control n = 16 | ↑ NOX; ↑ ROS; ↓ CAT; ↓ GSH; ↓ vitamin E; ↓ GPX | ↑ MDA | [36] | |
| Psoriasis n = 50 Control n = 50 | ↓ SOD; ↓ vitamin E; ↓ vitamin A; ↓ Zn; ↓ Cu | ↑ MDA | [39] | |
| Psoriasis n = 29 Control n = 30 | ≈ native SH; ≈ total SH; ↑ SS; ≈ SS/total SH | [40] | ||
| Psoriasis n = 80 Control n = 80 | ≈ native SH; ↓ total SH; ≈ SS; ↑ SS/total SH | [41] | ||
| Psoriasis n = 92 Control n = 71 | ≈ native SH; ≈ total SH; ↑ SS; ↓ SS/total SH | ↑ TC; ↑ TG; ≈ HDL-chol; ≈ LDL-chol | [42] | |
| Psoriasis n = 32 Control n = 34 | ↓ G6PDH | ↑ ESP | [43] | |
| Psoriasis n = 100 Control n = 100 | ↓ SOD; ↓ CAT | ↑ TC; ↑ LDL-chol; ↓ HDL-chol; ↑ ApoB; ↑ Lp(a); ↑ TG | ↑ MDA; ↓ PON | [44] |
| Psoriasis n = 93 Control n = 60 | ↓ HDL-chol; ≈ LDL-chol; ≈ TG; ≈ TC; ↓ ox-LDL | [45] | ||
| Psoriasis n = 150 Control n = 150 | ↓ RGSH | ↓ HDL-chol; ≈ LDL-chol; ↑ TG; ↑ TC; ↑ ox-LDL; | ↑ MDA | [46] |
| Psoriasis n = 50 Control n = 50 | ↑ MDA | [47] | ||
| Psoriasis n = 34 Control n = 30 | ↓ SOD; ↑ CAT | ↑ MDA | [48] | |
| Psoriasis n = 60 Control n = 60 | ↓ GPX; ≈ CAT; ≈ SOD | ↑ TG; ↑ TC; ↑ LDH-chol; ≈ HDL-chol | ↑ MDA | [49] |
| Psoriasis n = 30 Control n = 30 | ↓ SOD; ↓ GPX | ↑ MDA | [50] | |
| Psoriasis n = 70 Control n = 30 | ↑ TG; ↑ TC; ↑ LDH-chol; ↑ VLDL-chol; ≈ HDL-chol | ↑ MDA | [51] | |
| Psoriasis n = 23 Control n = 23 | ↑ NO | ↑ MDA | [52] | |
| Psoriasis n = 50 Control n = 50 | ↓ SOD; ↓ GPX | ↑ TG; ↑ TC; ↑ LDH-chol; ↓ HDL-chol; ↑ VLDL-chol | ↑ MDA | [53] |
| Antioxidant/Design | Duration of Therapy | Characteristic | Target Sites | Antioxidant Capacity | References |
|---|---|---|---|---|---|
| Tablet containing 100 μg selenium + 1500 IU vitamin A + 90 mg vitamin C + 30 mg vitamin E; once daily | 4 weeks | Seven psoriatic patients, different severities | Plasma, erythrocytes | ≈ MDA; ≈ CAT; ≈ SOD | [48] |
| Epigallocatechin-3-gallate EGCG glycerin solvent (3%, 30 mg/mL, glycerin solvent: 50% glycerin, 50% normal saline) | 3 weeks | Imiquimod induced psoriasis-like BALB/c mice | Serum | ↑ SOD; ↑ CAT; ↓ MDA | [153] |
| 30 mg β-carotene + 27.5 mg zinc sulfate monohydrate + 0.2 mg monohydrated selenium dioxide + 2 mg manganese + 1 mg copper | 8 weeks | 33 patients, 20–60 years, chronic plaque psoriasis | Serum | ↓ MDA; ↑ TAC | [157] |
| 50 mg/d coenzyme Q10 (ubiquinone acetate) + 50 mg/d vitamin E (natural alpha-tocopherol) + 48 µg/d selenium (aspartate salt) dissolved in soy lecithin | 30–35 days | 58 patients, severe erythrodermic and arthropathic forms of psoriasis | Granulocytes, affected epidermis | ↑ SOD; ↑ CAT | [158] |
| Nigella sativa ointment (20% w/w); Nigella sativa capsule (500 mg seeds powder) | 12 weeks | 60 patients, 3 groups: Group 1 (control)—methotrexate tablets; Group 2—Nigella sativa ointment 2 times daily + Nigella sativa capsule 3 times daily; Group 3—methotrexate tablets + Nigella sativa (ointment + capsule). | Serum | ↓ MDA | [160] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Mieczan, T.; Wójcik, G. Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet. Nutrients 2020, 12, 1841. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061841
Winiarska-Mieczan A, Mieczan T, Wójcik G. Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet. Nutrients. 2020; 12(6):1841. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061841
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Tomasz Mieczan, and Grzegorz Wójcik. 2020. "Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet" Nutrients 12, no. 6: 1841. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061841






